Abstract
Background and objectives
Pulse pressure has been shown as a risk factor for mortality in patients on maintenance hemodialysis (MHD). However, the effect of change in pulse pressure during hemodialysis on survival in a large cohort of patients on MHD has not been sufficiently investigated.
Design, setting, participants, & measurements
This study examined the association between time-varying Δ pulse pressure (postdialysis minus predialysis pulse pressure) and mortality in a cohort of 98,577 patients on MHD (July 2001–June 2006) using Cox proportional hazard models with restricted cubic splines.
Results
The average patient age was 62 years old; among the patients, 33% were black and 59% had diabetes. During 134,814 patient-years of at-risk time, 16,054 (16%) patients died, with 6827 (43%) of the deaths caused by cardiovascular causes. In the models including adjustment for either predialysis systolic BP or mean arterial BP, there was a U-shaped association between change in pulse pressure during hemodialysis and all-cause mortality. In the systolic BP plus case mix plus malnutrition-inflammation complex syndrome–adjusted model, large declines in pulse pressure (>–25 mmHg) and increases in pulse pressure >5 mmHg were associated with higher all-cause mortality (reference: ≥–5 to <5 mmHg): hazard ratios (95% confidence intervals [95% CIs]) for change pulse pressures of <–25, ≥–25 to <–15, ≥–15 to <–5, 5 to <15, 15 to <25, and ≥25 mmHg were 1.21 (95% CI, 1.14 to 1.29), 1.03 (95% CI, 0.97 to 1.10), 1.01 (95% CI, 0.96 to 1.06), 1.06 (95% CI, 1.01 to 1.11), 1.17 (95% CI, 1.11 to 1.24), and 1.15 (95% CI, 1.08 to 1.23), respectively. The U-shaped association was observed with cardiovascular death.
Conclusions
Modest reductions in pulse pressure after hemodialysis are associated with the greatest survival, whereas large declines or rises in pulse pressure are related to higher mortality. Trials determining how to modify pulse pressure response to improve survival in the hemodialysis population are indicated.
Keywords: survival, hemodialysis, hemodynamics and vascular regulation
Introduction
Increased pulse pressure (PP), as a surrogate of vascular stiffness, is an independent predictor of cardiovascular events, all-cause mortality, and cardiovascular mortality observed in the general, elderly, and hypertensive populations (1–5). At any mean arterial BP (MAP) level, patients on maintenance hemodialysis (MHD) have higher PP values than age-matched control subjects with normal renal function (6). Both increased arterial stiffness and PP have been associated with higher risks of cardiovascular events, all-cause mortality, and cardiovascular death in patients on MHD (7–12).
Cardiovascular disease is the leading cause of mortality in patients on MHD (13). Vascular changes, including atherosclerosis and arteriosclerosis, contribute to increased cardiovascular mortality in this population (14,15). Atherosclerosis is associated with increased arterial intima-media thickness leading to luminal obstruction with ischemic events. Arteriosclerosis results in arterial stiffening, increased pulse wave velocity (PWV), systolic BP (SBP), and PP, leading to left ventricular hypertrophy (LVH) and reduced coronary perfusion (16–19).
In patients on MHD, hypervolemia may play an important role on changes in pulse pressure (∆PP) (20). Volume overload has already been associated with LVH and mortality in patients on MHD (21–23). Combined volume correction with angiotensin-converting enzyme inhibition has been shown to improve aortic PWV in patients on MHD (24). Reductions in PWV may improve survival of patients on MHD (25). Decreased PP during hemodialysis has been demonstrated to be associated with lower risks of hospitalization or death (26). Previous observations are limited by regression models that assume a linear relationship between PP change and mortality. Therefore, they may be underpowered for detecting a U-shaped association of high mortality with either increases or decreases in PP (26). Both large declines and any rises in SBP during hemodialysis have been linked to increased mortality (27). The association between PP change during hemodialysis and mortality has not been sufficiently studied in large cohorts. We hypothesized that any increases or declines in PP during hemodialysis were associated with higher all-cause and cardiovascular mortality in addition to predialysis SBP levels in a large nationally representative cohort of patients on MHD.
Materials and Methods
Study Population and Data
We extracted and examined data from all patients receiving hemodialysis treatment between July 2001 and June 2006 in any of the 580 United States outpatient dialysis facilities of DaVita. The baseline quarter for each patient was the earliest calendar quarter in which the patient’s hemodialysis duration was >90 days. Of the 124,277 patients aged 18–99 years old who underwent hemodialysis for >90 days without modality switches during the study period, patients with missing BP measures (n=24,545), those with outliers of BP values (<0.25th or >99.75th percentiles; n=481), or those with outliers of PP values (<0.25th or >99.75th percentiles; n=207) were excluded, resulting in 99,044 patients for PP analysis (Supplemental Figure 1). The study was approved by the institutional review committees of the Los Angeles Biomedical Research Institute at Harbor–UCLA Medical Center and DaVita Clinical Research. The requirement for a written consent was exempted because of the large sample size, patient anonymity, and nonintrusive nature of the research.
The creation of the DaVita hemodialysis patient cohort has been described previously (28). All repeated clinical and laboratory measurements for each patient were averaged during any calendar quarters (i.e., over a 13-week interval). Clinical and laboratory parameters for each patient were obtained during the cohort period (July 1, 2001–June 30, 2006), and patients were followed for outcomes until June 30, 2006. Dialysis duration was defined as the duration of time between the first day of hemodialysis treatment and the day that the patient entered the cohort. Demographic data were obtained from the DaVita database. History of preexisting comorbid conditions and tobacco smoking were obtained by linking the DaVita database to the data from the Medical Evidence Form 2728 from the US Renal Data System. Available preexisting comorbidities were grouped into nine categories: ischemic heart disease (IHD), congestive heart failure (CHF), other cardiac diseases, hypertension, cerebrovascular disease, peripheral vascular disease, chronic obstructive pulmonary disease, cancer, and nonambulatory state.
Outcome Measures
All-cause mortality was defined by date of death if it occurred during the cohort period (July 1, 2001–June 30, 2006). The recorded causes of death were obtained from the US Renal Data System, and cardiovascular death was defined as death because of myocardial infarction, cardiac arrest, heart failure, cerebrovascular accident, and other cardiac diseases. Patients who received a kidney transplant or were lost to follow-up were censored at the time of the event.
Main Predictors
Pre- and postdialysis BPs with patients in a sitting position were measured during every hemodialysis session using a digital monitor with automatically inflated BP cuffs attached to a hemodialysis machine on the basis of the standard protocol of hemodialysis units. All recorded BP values were captured electronically within the database. All repeated BP values for each patient were averaged over each 13-week calendar quarter and used in analysis to minimize measurement variability. PP was quantified as the difference between the mean SBP and mean diastolic BP (DBP) for each calendar quarter. ∆PP was defined as postdialysis PP minus predialysis PP. Time-varying ∆PP for each patient was used in our analyses. We divided ∆PP levels a priori into seven categories (<–25, ≥–25 to <–15, ≥–15 to <–5, ≥–5 to<5, 5 to <15, 15 to <25, and ≥ 25 mmHg). The ∆PP category of ≥–5 to <5 mmHg was designated as the reference group.
Clinical and Laboratory Measures
MAP was calculated using the following equation: MAP=[(2×DBP)+SBP]/3′. Pre- and postdialysis body weights were recorded at each hemodialysis session. The volume of fluid removed during hemodialysis (ultrafiltration percentage [%UF]) was calculated as follows: %UF=[(predialysis weight–postdialysis weight)/predialysis weight]×100, where weights were measured in kilograms. Blood samples were drawn using uniform techniques in all DaVita dialysis clinics and were transported to the DaVita Laboratory in Deland, Florida, usually within 24 hours. All laboratory values were measured using automated and standardized methods in the DaVita Laboratory. Most laboratory parameters were measured monthly, including complete blood cell counts and serum levels of urea nitrogen, creatinine, albumin, calcium, phosphorus, bicarbonate, and total iron-binding capacity (TIBC). Serum ferritin levels were measured at least quarterly. Corrected serum calcium levels were calculated as follows: corrected calcium (mg/dl)={0.8×[4−serum albumin (g/dl)]}+serum calcium (mg/dl). Most blood samples were collected before hemodialysis, except for postdialysis serum urea nitrogen, to calculate urea kinetics.
Statistical Analyses
We evaluated the association of quarterly ∆PP as a main predictor with all-cause and cardiovascular mortality as outcomes using time-varying Cox proportional hazard regression models and restricted cubic splines. To minimize the influence of outliers, patients with outliers (<1st or >99th percentiles) of ∆PP (n=467) were excluded from the analyses. Therefore, the final study population consisted of 98,577 patients for the analyses (Supplemental Figure 1). The two separate adjusted models, including either predialysis SBP or predialysis MAP, were performed. For each model, three levels of multivariable adjustment were examined (1): minimally adjusted models that included the main predictors (∆PP), entry calendar quarter, and predialysis SBP or MAP; (2) case mix–adjusted models that included covariates in the minimally adjusted models plus age, sex, race/ethnicity (non-Hispanic whites, blacks, Hispanics, Asians, and others), dialysis duration categories (3 to <6 months, 6 to <24 months, 2 to <5 years, and ≥5 years), presence of diabetes, nine preexisting comorbidities, history of tobacco smoking, primary insurance (Medicare, Medicaid, private, and others), types of vascular access (arteriovenous fistula, arteriovenous graft, and catheter), dialysis dose as indicated by single-pool Kt/V (spKt/V), and %UF; and (3) case mix and malnutrition-inflammation complex syndrome (MICS)-adjusted models that included all of the covariates in the case mix models plus body mass index (BMI) and serum levels of albumin, creatinine, TIBC, ferritin, calcium, phosphorus, bicarbonate, hemoglobin, peripheral white blood cell count, and lymphocyte percentage. Time-varying covariates included predialysis SBP or MAP, %UF, spKt/V, BMI, and serum levels of albumin, creatinine, TIBC, ferritin, calcium, phosphorus, bicarbonate, hemoglobin, white blood cell count, and lymphocyte percentage. Fixed baseline covariates included age, sex, race/ethnicity, dialysis duration categories, comorbidities, primary insurance, and types of vascular access.
Stratified analysis by predialysis SBP (<120, 120 to <140, 140 to <160, and ≥160 mmHg) was performed to evaluate for the effect modification. Sensitivity analyses using the Cox model were also conducted in subgroups of patients on the basis of baseline age, sex, race/ethnicity, presence or absence of diabetes, IHD, CHF, dialysis duration, and baseline BMI categories. An additional analysis adjusted for either predialysis PP or postdialysis PP was performed. Missing values of time-varying covariates were imputed using the values in the previous quarter, whereas missing data on fixed baseline covariates were imputed by the means or medians of the existing values as appropriate. We reported P values from two-sided tests with a significance level set to 0.05. All statistical analyses were performed using Stata version 12.1 (Stata, College Station, TX).
Results
Cohort Description
The baseline characteristics of the 98,577 hemodialysis patients stratified by ∆PP during hemodialysis are summarized in Table 1. The mean±SD patient age at baseline was 62±15 years; 45% of the patients were women, 33% were black, and 59% had diabetes. The mean±SD pre- and postdialysis PPs at baseline were 73±17 and 68±17 mmHg, respectively. Compared with patients with ∆PP of ≥–5 to < 5 mmHg, patients with ∆PP <–5 and >5 mmHg had a higher prevalence of diabetes, IHD, and CHF. Patients with greater reductions in PP during hemodialysis were more likely to have higher predialysis SBP and MAP, but lower postdialysis SBP and MAP. The amount of ultrafiltration per session and spKt/V negatively correlated with ∆PP levels. During 134,814 patient-years of at-risk time, 16,054 (16%) patients died, with 6827 (43%) deaths caused by cardiovascular causes. Crude all-cause and cardiovascular mortality rates were 119 per 1000 patient-years (95% confidence interval [95% CI], 117 to 121) and 51 per 1000 patient-years (95% CI, 49 to 52), respectively.
Table 1.
Baseline characteristics of 98,577 hemodialysis patients stratified by change in pulse pressure
| Variables | All | ∆PP (mmHg) | P Value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| <–25 | ≥–25 to <–15 | ≥–15 to <–5 | ≥–5 to <5 | 5 to <15 | 15 to <25 | ≥25 | |||
| n (%) | 98,577 (100) | 9038 (9) | 12,958 (13) | 24,424 (25) | 27,200 (28) | 15,406 (16) | 6177 (6) | 3374 (3) | |
| Age (y) | 62±15 | 63±14 | 62±14 | 62±15 | 61±16 | 62±16 | 63±15 | 63±15 | 0.04 |
| Women | 45 | 50 | 47 | 44 | 42 | 44 | 46 | 49 | <0.001 |
| Race/ethnicity | |||||||||
| White | 42 | 44 | 42 | 41 | 41 | 43 | 46 | 45 | 0.001 |
| Black | 33 | 31 | 32 | 33 | 34 | 33 | 31 | 34 | 0.02 |
| Hispanic | 15 | 15 | 15 | 15 | 15 | 14 | 14 | 14 | 0.01 |
| Asian | 3 | 3 | 4 | 3 | 3 | 3 | 3 | 2 | <0.001 |
| Others | 7 | 7 | 7 | 8 | 7 | 7 | 6 | 5 | <0.001 |
| HD duration | |||||||||
| 3 to <6 mo | 57 | 56 | 54 | 55 | 57 | 60 | 63 | 65 | <0.001 |
| 6 to <24 mo | 18 | 19 | 19 | 19 | 18 | 17 | 16 | 15 | <0.001 |
| 2 to <5 y | 16 | 17 | 18 | 17 | 16 | 15 | 14 | 13 | <0.001 |
| ≥5 y | 9 | 8 | 9 | 9 | 9 | 8 | 7 | 7 | <0.001 |
| Comorbidities | |||||||||
| DM | 59 | 68 | 64 | 58 | 55 | 58 | 60 | 62 | <0.001 |
| IHD | 22 | 22 | 21 | 21 | 21 | 22 | 24 | 24 | <0.001 |
| Cancer | 4.6 | 4.1 | 4.1 | 4.4 | 4.6 | 5 | 4.9 | 5.3 | <0.001 |
| CHF | 28 | 29 | 28 | 27 | 27 | 28 | 31 | 30 | 0.002 |
| COPD | 6 | 6 | 6 | 6 | 6 | 6 | 7 | 6 | 0.01 |
| CVD | 7 | 8 | 8 | 7 | 7 | 8 | 8 | 8 | 0.43 |
| Hypertension | 80 | 83 | 82 | 80 | 79 | 80 | 81 | 83 | 0.001 |
| Other cardiac diseases | 6 | 5 | 5 | 5 | 6 | 6 | 6 | 6 | <0.001 |
| PVD | 11 | 13 | 12 | 11 | 11 | 12 | 13 | 13 | 0.20 |
| Nonambulatory state | 2.8 | 2.7 | 2.6 | 2.7 | 2.8 | 3.3 | 3 | 3.4 | <0.001 |
| Current smoking | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 0.15 |
| Insurance | |||||||||
| Medicare | 68 | 69 | 69 | 68 | 68 | 69 | 69 | 69 | 0.23 |
| Medicaid | 6 | 5 | 5 | 6 | 6 | 6 | 6 | 6 | <0.001 |
| Private | 9 | 4 | 9 | 12 | 12 | 7 | 4 | 1 | <0.001 |
| Others | 17 | 22 | 17 | 14 | 14 | 18 | 21 | 24 | 0.01 |
| Vascular access | |||||||||
| AVF | 30 | 29 | 31 | 31 | 30 | 28 | 25 | 25 | <0.001 |
| AVG | 31 | 30 | 33 | 33 | 32 | 29 | 27 | 24 | <0.001 |
| Catheter | 39 | 41 | 36 | 36 | 38 | 43 | 48 | 51 | <0.001 |
| Pre-HD BP (mmHg) | |||||||||
| Systolic BP | 151±24 | 172±22 | 162±21 | 153±22 | 146±23 | 143±24 | 140±24 | 137±23 | <0.001 |
| Diastolic BP | 78±15 | 80±16 | 80±15 | 78±14 | 77±14 | 77±15 | 77±16 | 77±17 | <0.001 |
| PP | 73±17 | 92±15 | 82±15 | 75±15 | 69±15 | 66±16 | 64±16 | 60±16 | <0.001 |
| Mean arterial BP | 102±17 | 110±17 | 107±16 | 103±15 | 100±16 | 99±17 | 98±17 | 97±18 | <0.001 |
| Post-HD BP (mmHg) | |||||||||
| Systolic BP | 141±23 | 129±19 | 134±20 | 136±21 | 141±22 | 149±24 | 157±24 | 164±24 | <0.001 |
| Diastolic BP | 73±14 | 72±14 | 72±13 | 72±13 | 73±13 | 74±15 | 74±16 | 73±17 | <0.001 |
| Pulse pressure | 68±17 | 57±15 | 62±15 | 64±15 | 68±15 | 75±16 | 83±16 | 91±16 | <0.001 |
| Mean arterial BP | 95±16 | 91±14 | 92±14 | 93±14 | 96±15 | 99±17 | 102±17 | 103±18 | <0.001 |
| ∆PP (mmHg) | −5±16 | −35±7 | −20±3 | −10±3 | −1±3 | 9±3 | 19±3 | 31±5 | <0.001 |
| Time on dialysis (min) | 214±28 | 214±28 | 214±28 | 215±28 | 214±28 | 214±27 | 214±28 | 213±28 | 0.35 |
| Ultrafiltration (kg/session) | 2.6±1.2 | 2.9±1.3 | 2.8±1.2 | 2.7±1.2 | 2.6±1.2 | 2.5±1.2 | 2.4±1.3 | 2.3±1.3 | <0.001 |
| Ultrafiltration (%)a | 3.4±1.5 | 3.6±1.5 | 3.6±1.4 | 3.5±1.4 | 3.4±1.5 | 3.2±1.6 | 3.1±1.6 | 3.0±1.6 | <0.001 |
| BMI (kg/m2)b | 26.9±6.1 | 27.9±6.5 | 27.5±6.3 | 27.0±6.1 | 26.6±6.0 | 26.2±5.8 | 26.3±6.1 | 26.5±6.2 | <0.001 |
| Laboratory measures (baseline) | |||||||||
| Kt/V (single pool) | 1.55±0.30 | 1.56±0.30 | 1.56±0.30 | 1.55±0.30 | 1.54±0.31 | 1.53±0.31 | 1.54±0.31 | 1.53±0.31 | <0.001 |
| Albumin (g/dl) | 3.7±0.5 | 3.7±0.4 | 3.7±0.4 | 3.7±0.4 | 3.7±0.5 | 3.6±0.5 | 3.6±0.5 | 3.6±0.5 | <0.001 |
| Creatinine (mg/dl) | 8.0±3.2 | 7.7±2.9 | 8.1±3.1 | 8.3±3.2 | 8.1±3.3 | 7.6±3.1 | 7.1±3.0 | 7.0±2.8 | <0.001 |
| TIBC (mg/dl) | 208±45 | 212±43 | 210±43 | 208±44 | 207±45 | 206±46 | 208±48 | 207±47 | <0.001 |
| Ferritin (ng/ml)c | 390 (190–683) | 373 (184–663) | 414 (198–702) | 410 (200–698) | 391 (189–687) | 377 (183–674) | 349 (173–640) | 341 (174–619) | <0.001 |
| Bicarbonate (mg/dl) | 22.1±3.2 | 22.2±3.2 | 22.0±3.1 | 21.9±3.1 | 22.1±3.1 | 22.4±3.2 | 22.6±3.1 | 22.7±3.2 | <0.001 |
| Calcium (mg/dl) | 9.5±0.7 | 9.5±0.7 | 9.6±0.7 | 9.6±0.7 | 9.5±0.7 | 9.5±0.7 | 9.5±0.7 | 9.5±0.6 | <0.001 |
| Phosphorus (mg/dl) | 5.6±1.5 | 5.7±1.4 | 5.7±1.4 | 5.7±1.5 | 5.6±1.5 | 5.5±1.5 | 5.4±1.5 | 5.4±1.5 | <0.001 |
| Hemoglobin (g/dl) | 12.1±1.3 | 12.3±1.3 | 12.3±1.2 | 12.2±1.3 | 12.1±1.3 | 12.0±1.4 | 12.0±1.4 | 12.1±1.4 | <0.001 |
| WBC (×103/µl) | 7.38±2.43 | 7.51±2.36 | 7.42±2.29 | 7.35±2.42 | 7.31±2.46 | 7.36±2.41 | 7.52±2.60 | 7.64±2.62 | 0.04 |
| Lymphocyte (%) | 20.6±7.8 | 20.7±7.4 | 21.1±7.6 | 21.0±7.8 | 20.6±8.0 | 20.1±7.8 | 19.8±7.6 | 19.6±7.7 | <0.001 |
Data are presented as percentages, means±SDs, or as otherwise indicated. The P value for trend shows the differences in each variable across ∆PP categories. HD, hemodialysis; DM, diabetes mellitus; IHD, ischemic heart disease; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; CVD, cerebrovascular disease; PVD, peripheral vascular disease; AVF, arteriovenous fistula; AVG, arteriovenous graft; PP, pulse pressure; ∆PP, postdialysis pulse pressure minus predialysis pulse pressure; BMI, body mass index; TIBC, total iron-binding capacity; WBC, white blood cells.
Calculated as [(predialysis weight−postdialysis weight)/predialysis weight]×100.
Calculated using postdialysis weight and height.
Median (interquartile range) is used.
Association between ∆PP and Mortality
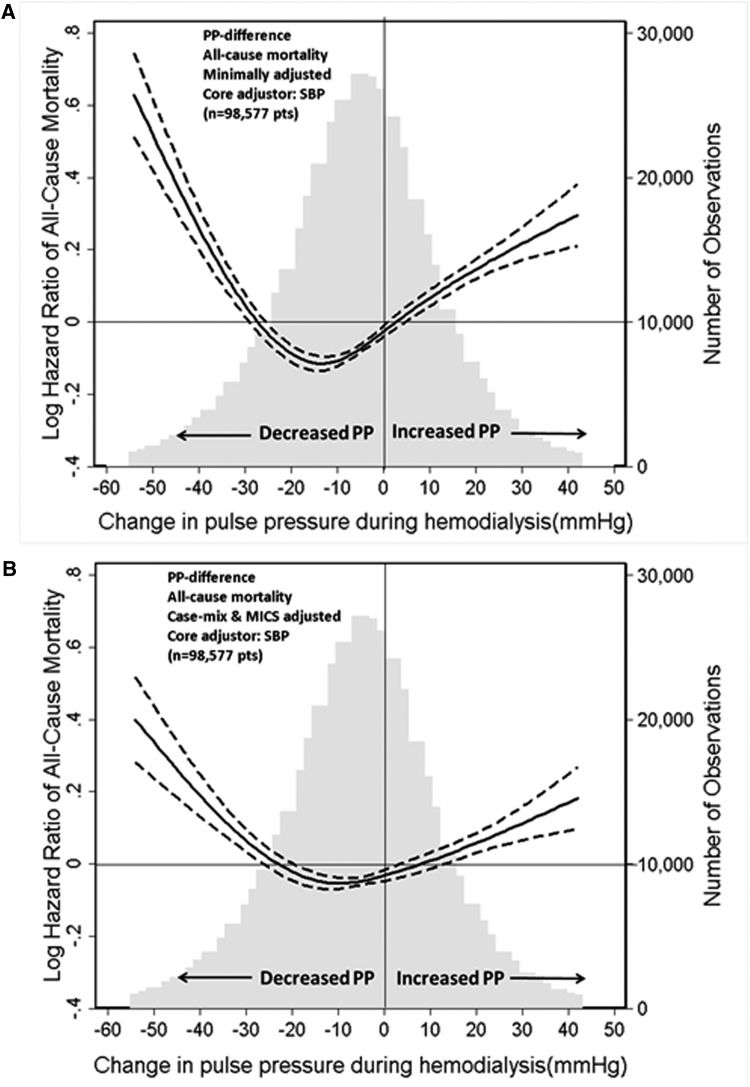
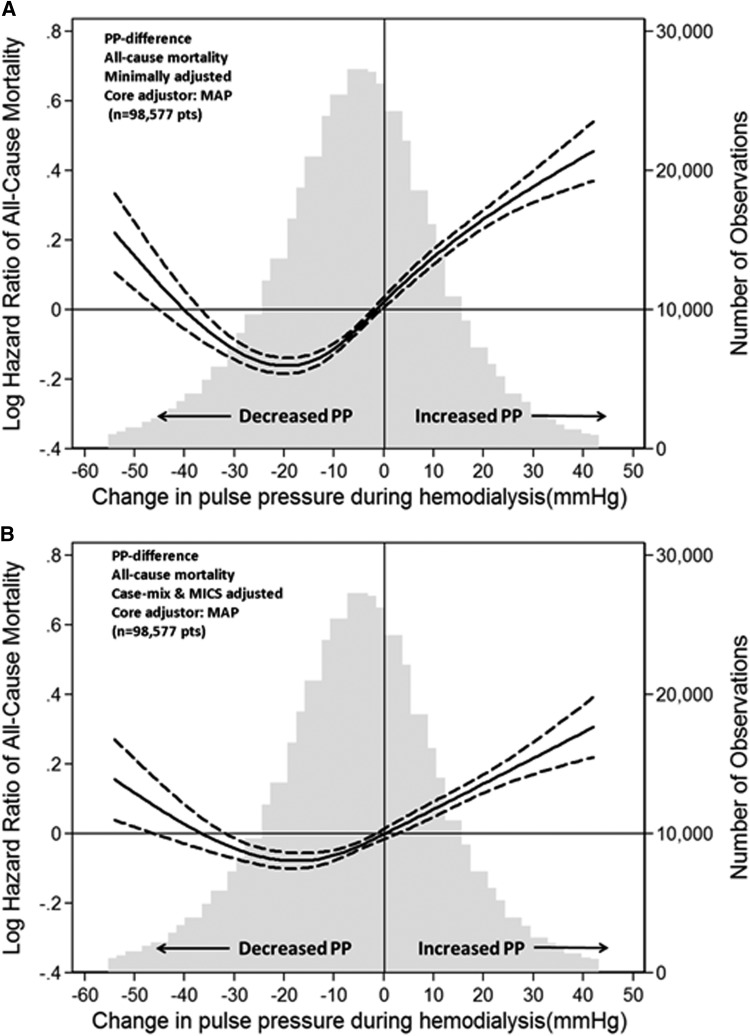
In the minimally adjusted models including either predialysis SBP or predialysis MAP, there was a U-shaped association between ∆PP and all-cause mortality (Figures 1 and 2). Large reductions in PP and increased PP during hemodialysis were associated with higher all-cause mortality. The U-shaped relationship between ∆PP and all-cause mortality persisted in the case mix (Supplemental Figure 2) and case mix plus MICS-adjusted models (Figures 1 and 2). The predialysis SBP-adjusted and MAP-adjusted hazard ratios of all-cause mortality associated with ∆PP during hemodialysis are shown in Table 2. In the SBP plus case mix plus MICS-adjusted model, compared with patients in the reference category of ∆PP ≥–5 to <5 mmHg, death risks were higher in patients with ∆PP<–25 mmHg (HR, 1.21; 95% CI, 1.14 to 1.29), 5 to <15 mmHg (HR, 1.06; 95% CI, 1.01 to 1.11), 15 to <25 mmHg (HR, 1.17; 95% CI, 1.11 to 1.24), and ≥25 mmHg (HR, 1.15; 95% CI, 1.08–1.23), respectively (Table 2). A similar trend was observed with cardiovascular mortality (Supplemental Figure 3, Table 3).
Figure 1.
SBP-adjusted hazard ratio for the association between change in pulse pressure during hemodialysis and all-cause mortality. Minimally SBP-adjusted model (A) and SBP plus case-mix plus MICS-adjusted model (B). Dashed lines represent 95% confidence interval. Change in PP was defined as postdialysis PP minus predialysis PP. Minimally SBP-adjusted model included adjustment for entry calendar quarter and predialysis SBP. SBP plus case mix plus MICS-adjusted model included covariates in the minimally SBP-adjusted model plus age, sex, race/ethnicity, presence of diabetes mellitus, nine preexisting comorbidities, history of tobacco smoking, dialysis duration categories, primary insurance, types of vascular access, dialysis dose as indicated by single pool Kt/V, ultrafiltration percentage, body mass index, serum levels of albumin, creatinine, total iron-binding capacity, ferritin, calcium, phosphorus, bicarbonate, hemoglobin, blood white blood cells, and lymphocyte percentage. MICS, malnutrition-inflammation complex syndrome; PP, pulse pressure; SBP, systolic BP.
Figure 2.
MAP-adjusted hazard ratio for the association between change in pulse pressure during hemodialysis and all-cause mortality. Minimally MAP-adjusted model (A) and MAP plus case-mix plus MICS-adjusted model (B). Dashed lines represent 95% confidence interval. Change in PP was defined as postdialysis PP minus predialysis PP. Minimally MAP-adjusted model included adjustment for entry calendar quarter and predialysis MAP. MAP plus case mix plus MICS-adjusted model included covariates in the minimally MAP-adjusted model plus age, sex, race/ethnicity, presence of diabetes mellitus, nine preexisting comorbidities, history of tobacco smoking, dialysis duration categories, primary insurance, types of vascular access, dialysis dose as indicated by single pool Kt/V, ultrafiltration percentage, body mass index, serum levels of albumin, creatinine, total iron-binding capacity, ferritin, calcium, phosphorus, bicarbonate, hemoglobin, blood white blood cells, and lymphocyte percentage. MAP, mean arterial BP; MICS, malnutrition-inflammation complex syndrome; PP, pulse pressure.
Table 2.
Hazard ratios (95% confidence intervals) for the association between change in pulse pressure categories (reference: ≥–5 to <5 mmHg) and all-cause mortality using time-varying Cox regression analyses
| ∆ Pulse Pressure (mmHg) | Minimally SBP Adjusted (n=98,577) | SBP and Case Mix Adjusted (n=98,577) | SBP and Case Mix and MICS Adjusted (n=98,577) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P Value | HR | 95% CI | P Value | HR | 95% CI | P Value | |
| <–25 | 1.26 | 1.18 to 1.34 | <0.001 | 1.12 | 1.05 to 1.19 | <0.001 | 1.21 | 1.14 to 1.29 | <0.001 |
| ≥–25 to <–15 | 1.00 | 0.94 to 1.06 | 0.92 | 0.95 | 0.89 to 1.00 | 0.06 | 1.03 | 0.97 to 1.10 | 0.27 |
| ≥–15 to <–5 | 0.95 | 0.90 to 0.99 | 0.02 | 0.93 | 0.89 to 0.98 | 0.01 | 1.01 | 0.96 to 1.06 | 0.74 |
| ≥–5 to <5 | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference |
| 5 to <15 | 1.13 | 1.08 to 1.19 | <0.001 | 1.10 | 1.05 to 1.16 | <0.001 | 1.06 | 1.01 to 1.11 | 0.02 |
| 15 to <25 | 1.30 | 1.23 to 1.37 | <0.001 | 1.24 | 1.18 to 1.32 | <0.001 | 1.17 | 1.11 to 1.24 | <0.001 |
| ≥25 | 1.27 | 1.18 to 1.35 | <0.001 | 1.20 | 1.12 to 1.28 | <0.001 | 1.15 | 1.08 to 1.23 | <0.001 |
| ∆ Pulse Pressure (mmHg) | Minimally MAP Adjusted (n=98,577) | MAP and Case Mix Adjusted (n=98,577) | MAP and Case Mix and MICS Adjusted (n=98,577) | ||||||
| HR | 95% CI | P Value | HR | 95% CI | P Value | HR | 95% CI | P Value | |
| <-25 | 0.98 | 0.92 to 1.04 | 0.43 | 0.90 | 0.85 to 0.96 | 0.001 | 1.03 | 0.97 to 1.10 | 0.34 |
| ≥–25 to <–15 | 0.88 | 0.83 to 0.93 | <0.001 | 0.84 | 0.79 to 0.89 | <0.001 | 0.95 | 0.89 to 1.00 | 0.07 |
| ≥–15 to <–5 | 0.90 | 0.85 to 0.94 | <0.001 | 0.89 | 0.84 to 0.93 | <0.001 | 0.97 | 0.92 to 1.02 | 0.18 |
| ≥–5 to <5 | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference |
| 5 to <15 | 1.17 | 1.11 to 1.22 | <0.001 | 1.15 | 1.09 to 1.20 | <0.001 | 1.09 | 1.04 to 1.14 | <0.001 |
| 15 to <25 | 1.37 | 1.29 to 1.44 | <0.001 | 1.32 | 1.25 to 1.40 | <0.001 | 1.23 | 1.16 to 1.30 | <0.001 |
| ≥25 | 1.39 | 1.30 to 1.49 | <0.001 | 1.33 | 1.25 to 1.43 | <0.001 | 1.24 | 1.16 to 1.33 | <0.001 |
Minimally adjusted model included adjustment for entry calendar quarter and predialysis SBP or predialysis MAP. Case mix–adjusted model included adjustment for all covariates in the minimally adjusted model plus age, sex, race/ethnicity, presence of diabetes mellitus, nine preexisting comorbidities, history of tobacco smoking, dialysis duration categories, primary insurance, types of vascular access, dialysis dose as indicated by single pool Kt/V, and ultrafiltration percentage. Case mix and MICS adjusted for covariates in the case mix–adjusted model plus body mass index; serum levels of albumin, creatinine, total iron-binding capacity, ferritin, calcium, phosphorus, bicarbonate, hemoglobin, and white blood cells; and lymphocyte percentage. ∆ Pulse pressure, postdialysis pulse pressure minus predialysis pulse pressure; SBP, systolic BP; HR, hazard ratio; MAP, mean arterial BP; 95% CI, 95% confidence interval; MICS, malnutrition-inflammation complex syndrome.
Table 3.
Hazard ratios (95% confidence intervals) for the association of change in pulse pressure categories (reference: ≥–5 to <5 mmHg) with cardiovascular mortality using time-varying Cox regression analyses
| ∆ Pulse pressure (mmHg) | Minimally SBP Adjusted (n=98,577) | SBP and Case Mix Adjusted (n=98,577) | SBP and Case Mix and MICS Adjusted (n=98,577) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P Value | HR | 95% CI | P Value | HR | 95% CI | P Value | |
| <–25 | 1.12 | 1.02 to 1.23 | 0.02 | 0.99 | 0.90 to 1.09 | 0.90 | 1.07 | 0.97 to 1.18 | 0.18 |
| ≥–25 to <-15 | 0.86 | 0.78 to 0.94 | <0.001 | 0.81 | 0.74 to 0.89 | <0.001 | 0.88 | 0.80 to 0.96 | 0.01 |
| ≥–15 to <-5 | 0.85 | 0.79 to 0.92 | <0.001 | 0.84 | 0.78 to 0.91 | <0.001 | 0.90 | 0.84 to 0.97 | 0.01 |
| ≥–5 to <5 | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference |
| 5 to <15 | 1.06 | 0.99 to 1.14 | 0.10 | 1.04 | 0.97 to 1.12 | 0.28 | 1.00 | 0.93 to 1.07 | 1.00 |
| 15 to <25 | 1.30 | 1.19 to 1.41 | <0.001 | 1.25 | 1.15 to 1.36 | <0.001 | 1.18 | 1.08 to 1.28 | <0.001 |
| ≥25 | 1.12 | 1.01 to 1.24 | 0.04 | 1.06 | 0.95 to 1.18 | 0.29 | 1.02 | 0.92 to 1.14 | 0.71 |
| ∆ Pulse Pressure (mmHg) | Minimally MAP Adjusted (n=98,577) | MAP and Case Mix Adjusted (n=98,577) | MAP and Case Mix and MICS Adjusted (n=98,577) | ||||||
| HR | 95% CI | P Value | HR | 95% CI | P Value | HR | 95% CI | P Value | |
| <–25 | 0.90 | 0.82 to 0.99 | 0.03 | 0.82 | 0.75 to 0.90 | <0.001 | 0.92 | 0.84 to 1.01 | 0.09 |
| ≥–25 to <-15 | 0.77 | 0.71 to 0.85 | <0.001 | 0.73 | 0.67 to 0.80 | <0.001 | 0.81 | 0.74 to 0.89 | <0.001 |
| ≥–15 to <-5 | 0.82 | 0.76 to 0.88 | <0.001 | 0.80 | 0.75 to 0.87 | <0.001 | 0.87 | 0.81 to 0.93 | <0.001 |
| ≥–5 to <5 | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference |
| 5 to <15 | 1.09 | 1.02 to 1.17 | 0.02 | 1.07 | 1.00 to 1.15 | 0.05 | 1.02 | 0.95 to 1.10 | 0.52 |
| 15 to <25 | 1.36 | 1.25 to 1.47 | <0.001 | 1.32 | 1.21 to 1.43 | <0.001 | 1.23 | 1.13 to 1.34 | <0.001 |
| ≥25 | 1.22 | 1.09 to 1.35 | <0.001 | 1.17 | 1.05 to 1.30 | 0.005 | 1.09 | 0.98 to 1.22 | 0.10 |
Minimally adjusted model included adjustment for entry calendar quarter and predialysis SBP or predialysis MAP. Case mix–adjusted model included adjustment for all covariates in the minimally adjusted model plus age, sex, race/ethnicity, presence of diabetes mellitus, nine preexisting comorbidities, history of tobacco smoking, dialysis duration categories, primary insurance, types of vascular access, dialysis dose as indicated by single pool Kt/V, and ultrafiltration percentage. Case mix and MICS adjusted for covariates in the case mix–adjusted model plus body mass index, serum levels of albumin, creatinine, total iron-binding capacity, ferritin, calcium, phosphorus, bicarbonate, hemoglobin, and white blood cells, and lymphocyte percentage. ∆ Pulse pressure, postdialysis pulse pressure minus predialysis pulse pressure; SBP, systolic BP; HR, hazard ratio; MAP, mean arterial BP; 95% CI, 95% confidence interval; MICS, malnutrition-inflammation complex syndrome.
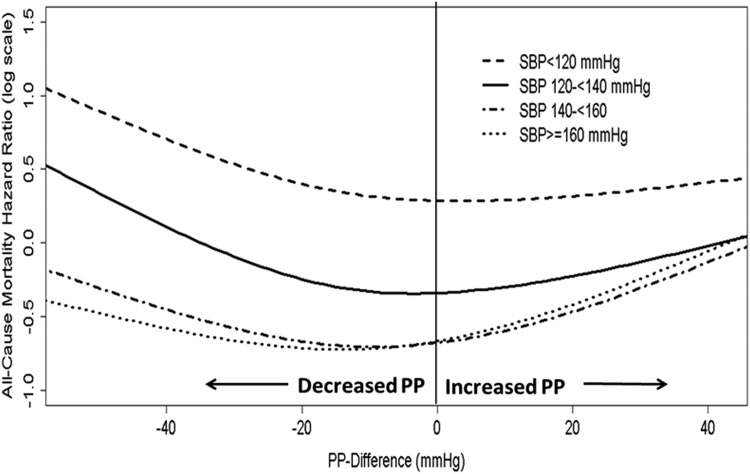
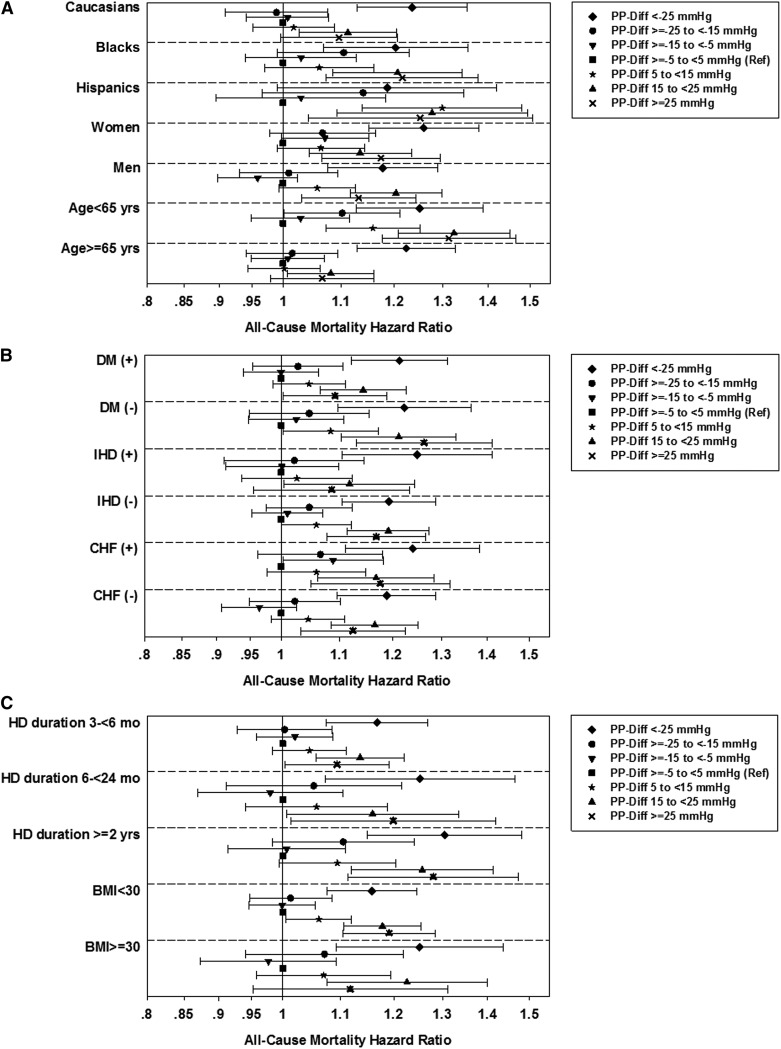
Sensitivity Analyses
In the case mix plus MICS-adjusted analyses stratified according to predialysis SBP, the U-shaped associations between ΔPP and all-cause mortality were present in the categories of predialysis SBP ≥120 mmHg. In the category of predialysis SBP <120 mmHg, only a decrease in PP during hemodialysis was associated with higher mortality (Figure 3). The P value for the interaction between SBP strata and ∆PP for all-cause mortality was <0.001. The U-shaped associations between ∆PP and all-cause mortality were also consistent in all subgroups of patients on the basis of demographics (baseline age, sex, race/ethnicity), presence or absence of diabetes, IHD, and CHF, dialysis duration, and baseline BMI categories (Figure 4). The results of the analyses conducted with and without imputation of missing covariates were essentially the same (Supplemental Table 1). The results did not change in analyses, including outliers <1st percentile and >99th percentile of ∆PP (Supplemental Table 2). The case mix and MICS-adjusted model, including predialysis PP as a covariate, showed the U-shaped association (Supplemental Figure 4A), whereas the model adjusted for postdialysis PP found that a decrease in PP during hemodialysis was associated with greater survival (Supplemental Figure 4B).
Figure 3.
Effect modification by predialysis SBP on the association between change in pulse pressure and all-cause mortality for case mix plus MICS–adjusted models. Change in PP was defined as postdialysis PP minus predialysis PP. Case mix plus MICS-adjusted model included adjustment for entry calendar quarter, age, sex, race/ethnicity, presence of diabetes mellitus, nine preexisting comorbidities, history of tobacco smoking, dialysis duration categories, primary insurance, types of vascular access, dialysis dose as indicated by single pool Kt/V, ultrafiltration percentage, body mass index, serum levels of albumin, creatinine, total iron-binding capacity, ferritin, calcium, phosphorus, bicarbonate, hemoglobin, blood white blood cells, and lymphocyte percentage. MICS, malnutrition-inflammation complex syndrome; PP, pulse pressure; SBP, systolic BP.
Figure 4.
Effect of race/ethnicity, sex, age, comorbidities, dialysis duration, and body mass index on the association between change in pulse pressure during hemodialysis and all-cause mortality. All-cause death hazard ratios (95% confidence intervals) comparing change in PP categories (Ref: ethnicity, sex, baseline age (A), presence or absence of DM, IHD, CHF (B), dialysis duration, and baseline body mass index categories (C) for SBP plus case mix plus MICS–adjusted models. The change in PP was defined as postdialysis PP minus predialysis PP. The model included adjustment for entry calendar quarter, predialysis systolic BP, age, sex, race/ethnicity, presence of diabetes mellitus, nine preexisting comorbidities, history of tobacco smoking, dialysis duration categories, primary insurance, types of vascular access, dialysis dose as indicated by single pool Kt/V, ultrafiltration percentage, body mass index, serum levels of albumin, creatinine, total iron-binding capacity, ferritin, calcium, phosphorus, bicarbonate, hemoglobin, and white blood cells, and lymphocyte percentage. CHF, chronic heart failure; Diff, difference; DM, diabetes mellitus; IHD, ischemic heart disease; MICS, malnutrition-inflammation complex syndrome; PP, pulse pressure; Ref, reference; SBP, systolic blood pressure.
Discussion
In this large cohort of 98,577 patients on MHD, we observed a U-shaped association between ∆PP during hemodialysis and mortality. Large declines in PP during hemodialysis were related to higher mortality, whereas moderate reductions in PP were associated with the greatest survival. These associations were observed in all demographic and clinical subgroups. Similar patterns of associations were observed with all-cause and cardiovascular mortality. To our knowledge this is the largest epidemiology study to examine the association of ∆PP over time and subsequent mortality in patients on MHD.
Although we have previously shown that large declines and any rises in SBP during hemodialysis have been linked to higher mortality (27), there may be added benefit in examining changes in intradialytic PP. In the general population, PP has been shown to be a predictor of adverse outcomes independent of SBP. For example, in a study of 69,989 subjects >50 years old, a higher absolute PP was associated with increased cardiovascular mortality even among patients with normal SBP (29). Several earlier studies have also shown that PP is a predictor of cardiovascular events and mortality in patients on MHD (6,11), and recent data suggest that PP may in fact be a better predictor of death and cardiovascular events than other BP parameters in the hemodialysis population (30).
Decreasing PP during hemodialysis has been shown to be associated with improved short-term outcomes. In a cohort of 438 hemodialysis patients, every 10 mmHg decline in PP during hemodialysis was associated with a 20% decreased hazard of death or hospitalization at 6 months (26). Greater reductions in PP induced by hemodialysis could be caused by higher fluid or sodium solute removal in these subjects. PP responses to hemodialysis are closely related to blood volume changes (31). Fluid removal lowers SBP more than DBP, leading to reductions in PP during hemodialysis (32,33). We found that a large reduction in PP was associated with higher mortality. In our study, patients with greater decline in PP after hemodialysis correlated with more fluid removal during hemodialysis. Higher ultrafiltration volumes have been associated with the presence and severity of hemodialysis-induced cardiac injury. Myocardial stunning leads to loss of systolic function and increased cardiac events and mortality (34). It is plausible that subsequent hemodialysis-associated cardiac ischemia might explain our findings. Decreased SBP and PP during hemodialysis were associated with improved arterial stiffness induced by a hemodialysis session (35). Reduction in aortic stiffness as measured by PWV in response to BP lowering has been shown to improve survival of patients on MHD (25). Molecules that may influence arterial function, such as endothelin, nitric oxide, and angiotensin II, are removed during hemodialysis, resulting in improved arterial stiffness. Tycho Vuurmans et al. (24) reported that combined volume correction with angiotensin-converting enzyme inhibition reduced aortic PWV. Several studies have demonstrated that a hemodialysis session led to an improvement in endothelial function (36–38).
Nevertheless, inability to decrease PP during hemodialysis may be the result of the presence of intrinsic vascular lesions. Increased arterial stiffness may be caused by structural changes of artery, which is less dependent on BP (39–43). Arterial stiffening may also result from high BP without structural changes and can be reversed with BP lowering. Past observations investigating the influence of hemodialysis on alterations in arterial compliance as reflected by the aortic augmentation index (AI) showed that patients whose AI remained abnormal after hemodialysis had larger hearts than those whose AIs normalized with hemodialysis. Persistently high AIs after hemodialysis might be explained by the presence of vascular structural damage (44). Vascular calcification has been associated with LVH (45,46), adverse cardiovascular outcomes, and mortality in patients on MHD (47,48).
The association between a rise in PP after hemodialysis and a higher risk of mortality was observed in our study. PP increase during hemodialysis could be a marker of vascular disease or could be caused by alteration in autonomic control. Elevated PP during hemodialysis in patients with peripheral vascular disease may be in response to volume depletion induced by hemodialysis caused by reduced autonomic peripheral control, reduced cardiac baroreflex, and lower sympathetic activity on BP and heart rate (49).
Several limitations of our study bear mention. First, this study was observational in nature, and no causality can be established from the results. Second, increased PP could reflect either decreased vascular compliance (17,19) or hypervolemia (20) in patients on MHD. More precise measurements of vascular stiffness (e.g., PWV, aortic compliance) and volume status (e.g., bioimpedance analysis) were not performed in our study because of limited feasibility of these investigations in the large study population. We lacked information on prescribed dialysate calcium, sodium, bicarbonate concentration, predialysis serum sodium concentration, ultrafiltration rate, and profile during hemodialysis; therefore, we could not determine whether these factors contributed to ∆PP during hemodialysis. Third, we had no information regarding the use of antihypertensive medications, which may confound the association between PP change and mortality. Fourth, we lacked data on patient compliance with hemodialysis treatments, and information on comorbidities was not updated during follow-up, which may result in residual confounding.
Despite the limitations, our study included (1) a large sample size of the cohort; (2) uniform administrative patient care in a large dialysis organization and laboratory measurements performed at a single facility; (3) use of 3-month averaged pre- and postdialysis SBP and DBP data from every single dialysis session to calculate ∆PP and averaged laboratory measures during any calendar quarter to minimize measurement variability; and (4) use of time-dependent survival models with multivariable adjustments for time-varying covariates as potential confounders.
In summary, our study demonstrated that modest declines in PP during hemodialysis were associated with improved survival, whereas any rises and large decreases in PP had higher mortality risk. ∆PP during hemodialysis could identify those patients most likely to respond favorably to a therapeutic PP reduction. Our study begs the question whether BP responses to hemodialysis are modifiable where targeting modest reductions in PP during hemodialysis can lead to improved patient survival.
Disclosures
None.
Supplementary Material
Acknowledgments
We thank DaVita Clinical Research for providing the clinical data, analysis, and review for this research project and for advancing the knowledge and practice of kidney care.
Supported by a research grant from the National Institute of Diabetes, Digestive and Kidney Disease of the National Institutes of Health (R01-DK078106 and K24-DK091419), a philanthropist grant from Mr. Harold Simmons, and a research grant from DaVita Clinical Research.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.09000914/-/DCSupplemental.
References
- 1.Benetos A, Rudnichi A, Safar M, Guize L: Pulse pressure and cardiovascular mortality in normotensive and hypertensive subjects. Hypertension 32: 560–564, 1998 [DOI] [PubMed] [Google Scholar]
- 2.Domanski M, Norman J, Wolz M, Mitchell G, Pfeffer M: Cardiovascular risk assessment using pulse pressure in the first national health and nutrition examination survey (NHANES I). Hypertension 38: 793–797, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Franklin SS, Khan SA, Wong ND, Larson MG, Levy D: Is pulse pressure useful in predicting risk for coronary heart Disease? The Framingham heart study. Circulation 100: 354–360, 1999 [DOI] [PubMed] [Google Scholar]
- 4.Millar JA, Lever AF, Burke V: Pulse pressure as a risk factor for cardiovascular events in the MRC Mild Hypertension Trial. J Hypertens 17: 1065–1072, 1999 [DOI] [PubMed] [Google Scholar]
- 5.Weiss A, Boaz M, Beloosesky Y, Kornowski R, Grossman E: Pulse pressure predicts mortality in elderly patients. J Gen Intern Med 24: 893–896, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tozawa M, Iseki K, Iseki C, Oshiro S, Yamazato M, Higashiuesato Y, Tomiyama N, Tana T, Ikemiya Y, Takishita S: Evidence for elevated pulse pressure in patients on chronic hemodialysis: A case-control study. Kidney Int 62: 2195–2201, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Amar J, Vernier I, Rossignol E, Bongard V, Arnaud C, Conte JJ, Salvador M, Chamontin B: Nocturnal blood pressure and 24-hour pulse pressure are potent indicators of mortality in hemodialysis patients. Kidney Int 57: 2485–2491, 2000 [DOI] [PubMed] [Google Scholar]
- 8.Blacher J, Guerin AP, Pannier B, Marchais SJ, Safar ME, London GM: Impact of aortic stiffness on survival in end-stage renal disease. Circulation 99: 2434–2439, 1999 [DOI] [PubMed] [Google Scholar]
- 9.Blacher J, Pannier B, Guerin AP, Marchais SJ, Safar ME, London GM: Carotid arterial stiffness as a predictor of cardiovascular and all-cause mortality in end-stage renal disease. Hypertension 32: 570–574, 1998 [DOI] [PubMed] [Google Scholar]
- 10.Foucan L, Deloumeaux J, Hue K, Foucan T, Blanchet-Deverly A, Merault H, Gabriel JM, Hiesse C: High pulse pressure associated with cardiovascular events in patients with type 2 diabetes undergoing hemodialysis. Am J Hypertens 18: 1457–1462, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Klassen PS, Lowrie EG, Reddan DN, DeLong ER, Coladonato JA, Szczech LA, Lazarus JM, Owen WF, Jr: Association between pulse pressure and mortality in patients undergoing maintenance hemodialysis. JAMA 287: 1548–1555, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Tozawa M, Iseki K, Iseki C, Takishita S: Pulse pressure and risk of total mortality and cardiovascular events in patients on chronic hemodialysis. Kidney Int 61: 717–726, 2002 [DOI] [PubMed] [Google Scholar]
- 13.Collins AJ, Foley RN, Herzog C, Chavers BM, Gilbertson D, Ishani A, Kasiske BL, Liu J, Mau LW, McBean M, Murray A, St Peter W, Guo H, Li Q, Li S, Li S, Peng Y, Qiu Y, Roberts T, Skeans M, Snyder J, Solid C, Wang C, Weinhandl E, Zaun D, Arko C, Chen SC, Dalleska F, Daniels F, Dunning S, Ebben J, Frazier E, Hanzlik C, Johnson R, Sheets D, Wang X, Forrest B, Constantini E, Everson S, Eggers PW, Agodoa L: Excerpts from the US Renal Data System 2009 Annual Data Report. Am J Kidney Dis 55[Suppl 1]: S1–S420, A6–A7, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.London GM, Guérin AP, Marchais SJ, Métivier F, Pannier B, Adda H: Arterial media calcification in end-stage renal disease: Impact on all-cause and cardiovascular mortality. Nephrol Dial Transplant 18: 1731–1740, 2003 [DOI] [PubMed] [Google Scholar]
- 15.London GM, Marchais SJ, Guerin AP: Arterial stiffness and function in end-stage renal disease. Adv Chronic Kidney Dis 11: 202–209, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Amann K: Media calcification and intima calcification are distinct entities in chronic kidney disease. Clin J Am Soc Nephrol 3: 1599–1605, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Barenbrock M, Spieker C, Laske V, Heidenreich S, Hohage H, Bachmann J, Hoeks AP, Rahn KH: Studies of the vessel wall properties in hemodialysis patients. Kidney Int 45: 1397–1400, 1994 [DOI] [PubMed] [Google Scholar]
- 18.London GM, Guerin AP, Marchais SJ, Pannier B, Safar ME, Day M, Metivier F: Cardiac and arterial interactions in end-stage renal disease. Kidney Int 50: 600–608, 1996 [DOI] [PubMed] [Google Scholar]
- 19.London GM, Marchais SJ, Safar ME, Genest AF, Guerin AP, Metivier F, Chedid K, London AM: Aortic and large artery compliance in end-stage renal failure. Kidney Int 37: 137–142, 1990 [DOI] [PubMed] [Google Scholar]
- 20.Yazici H, Oflaz H, Pusuroglu H, Tepe S, Dogan C, Basci A, Akkaya V, Yildiz A: Hypervolemia rather than arterial calcification and extracoronary atherosclerosis is the main determinant of pulse pressure in hemodialysis patients. Int Urol Nephrol 44: 1203–1210, 2012 [DOI] [PubMed] [Google Scholar]
- 21.Innes A, Charra B, Burden RP, Morgan AG, Laurent G: The effect of long, slow haemodialysis on patient survival. Nephrol Dial Transplant 14: 919–922, 1999 [DOI] [PubMed] [Google Scholar]
- 22.Ozkahya M, Ok E, Cirit M, Aydin S, Akçiçek F, Başçi A, Dorhout Mees EJ: Regression of left ventricular hypertrophy in haemodialysis patients by ultrafiltration and reduced salt intake without antihypertensive drugs. Nephrol Dial Transplant 13: 1489–1493, 1998 [DOI] [PubMed] [Google Scholar]
- 23.Ozkahya M, Ok E, Toz H, Asci G, Duman S, Basci A, Kose T, Dorhout Mees EJ: Long-term survival rates in haemodialysis patients treated with strict volume control. Nephrol Dial Transplant 21: 3506–3513, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Tycho Vuurmans JL, Boer WH, Bos WJ, Blankestijn PJ, Koomans HA: Contribution of volume overload and angiotensin II to the increased pulse wave velocity of hemodialysis patients. J Am Soc Nephrol 13: 177–183, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Guerin AP, Blacher J, Pannier B, Marchais SJ, Safar ME, London GM: Impact of aortic stiffness attenuation on survival of patients in end-stage renal failure. Circulation 103: 987–992, 2001 [DOI] [PubMed] [Google Scholar]
- 26.Inrig JK, Patel UD, Toto RD, Reddan DN, Himmelfarb J, Lindsay RM, Stivelman J, Winchester JF, Szczech LA: Decreased pulse pressure during hemodialysis is associated with improved 6-month outcomes. Kidney Int 76: 1098–1107, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park J, Rhee CM, Sim JJ, Kim YL, Ricks J, Streja E, Vashistha T, Tolouian R, Kovesdy CP, Kalantar-Zadeh K: A comparative effectiveness research study of the change in blood pressure during hemodialysis treatment and survival. Kidney Int 84: 795–802, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Streja E, Kovesdy CP, Molnar MZ, Norris KC, Greenland S, Nissenson AR, Kopple JD, Kalantar-Zadeh K: Role of nutritional status and inflammation in higher survival of African American and Hispanic hemodialysis patients. Am J Kidney Dis 57: 883–893, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thomas F, Blacher J, Benetos A, Safar ME, Pannier B: Cardiovascular risk as defined in the 2003 European blood pressure classification: The assessment of an additional predictive value of pulse pressure on mortality. J Hypertens 26: 1072–1077, 2008 [DOI] [PubMed] [Google Scholar]
- 30.Ishimitsu T, Nakano N, Sudo Y, Akashiba A, Takahashi T, Ohta S, Minami J, Matsuoka H: Predictive significance of blood pressure values for the incidence of cardiovascular events in chronic hemodialysis patients. Hypertens Res 31: 1703–1709, 2008 [DOI] [PubMed] [Google Scholar]
- 31.Bos WJ, Bruin S, van Olden RW, Keur I, Wesseling KH, Westerhof N, Krediet RT, Arisz LA: Cardiac and hemodynamic effects of hemodialysis and ultrafiltration. Am J Kidney Dis 35: 819–826, 2000 [DOI] [PubMed] [Google Scholar]
- 32.Agarwal R: Volume-associated ambulatory blood pressure patterns in hemodialysis patients. Hypertension 54: 241–247, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kovacic V, Roguljic L, Kovacic V, Bacic B, Bosnjak T: Ultrafiltration volume is associated with changes in blood pressure in chronically hemodialyzed patients. Ren Fail 25: 945–951, 2003 [DOI] [PubMed] [Google Scholar]
- 34.Burton JO, Jefferies HJ, Selby NM, McIntyre CW: Hemodialysis-induced cardiac injury: Determinants and associated outcomes. Clin J Am Soc Nephrol 4: 914–920, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mardare NG, Goldsmith DJ, Gusbeth-Tatomir P, Covic A: Intradialytic changes in reflective properties of the arterial system during a single hemodialysis session. Hemodial Int 9: 376–382, 2005 [DOI] [PubMed] [Google Scholar]
- 36.Covic A, Goldsmith DJ, Gusbeth-Tatomir P, Covic M: Haemodialysis acutely improves endothelium-independent vasomotor function without significantly influencing the endothelium-mediated abnormal response to a beta 2-agonist. Nephrol Dial Transplant 19: 637–643, 2004 [DOI] [PubMed] [Google Scholar]
- 37.Cross JM, Donald A, Vallance PJ, Deanfield JE, Woolfson RG, MacAllister RJ: Dialysis improves endothelial function in humans. Nephrol Dial Transplant 16: 1823–1829, 2001 [DOI] [PubMed] [Google Scholar]
- 38.Hand MF, Haynes WG, Webb DJ: Hemodialysis and L-arginine, but not D-arginine, correct renal failure-associated endothelial dysfunction. Kidney Int 53: 1068–1077, 1998 [DOI] [PubMed] [Google Scholar]
- 39.Guérin AP, London GM, Marchais SJ, Metivier F: Arterial stiffening and vascular calcifications in end-stage renal disease. Nephrol Dial Transplant 15: 1014–1021, 2000 [DOI] [PubMed] [Google Scholar]
- 40.London GM, Drueke TB: Atherosclerosis and arteriosclerosis in chronic renal failure. Kidney Int 51: 1678–1695, 1997 [DOI] [PubMed] [Google Scholar]
- 41.Mourad JJ, Girerd X, Boutouyrie P, Laurent S, Safar M, London G: Increased stiffness of radial artery wall material in end-stage renal disease. Hypertension 30: 1425–1430, 1997 [DOI] [PubMed] [Google Scholar]
- 42.Raggi P, Bellasi A, Ferramosca E, Islam T, Muntner P, Block GA: Association of pulse wave velocity with vascular and valvular calcification in hemodialysis patients. Kidney Int 71: 802–807, 2007 [DOI] [PubMed] [Google Scholar]
- 43.Sigrist MK, Taal MW, Bungay P, McIntyre CW: Progressive vascular calcification over 2 years is associated with arterial stiffening and increased mortality in patients with stages 4 and 5 chronic kidney disease. Clin J Am Soc Nephrol 2: 1241–1248, 2007 [DOI] [PubMed] [Google Scholar]
- 44.Covic A, Goldsmith DJ, Panaghiu L, Covic M, Sedor J: Analysis of the effect of hemodialysis on peripheral and central arterial pressure waveforms. Kidney Int 57: 2634–2643, 2000 [DOI] [PubMed] [Google Scholar]
- 45.Nitta K, Akiba T, Uchida K, Otsubo S, Otsubo Y, Takei T, Ogawa T, Yumura W, Kabaya T, Nihei H: Left ventricular hypertrophy is associated with arterial stiffness and vascular calcification in hemodialysis patients. Hypertens Res 27: 47–52, 2004 [DOI] [PubMed] [Google Scholar]
- 46.Yildiz A, Memisoglu E, Oflaz H, Yazici H, Pusuroglu H, Akkaya V, Erzengin F, Tepe S: Atherosclerosis and vascular calcification are independent predictors of left ventricular hypertrophy in chronic haemodialysis patients. Nephrol Dial Transplant 20: 760–767, 2005 [DOI] [PubMed] [Google Scholar]
- 47.Okuno S, Ishimura E, Kitatani K, Fujino Y, Kohno K, Maeno Y, Maekawa K, Yamakawa T, Imanishi Y, Inaba M, Nishizawa Y: Presence of abdominal aortic calcification is significantly associated with all-cause and cardiovascular mortality in maintenance hemodialysis patients. Am J Kidney Dis 49: 417–425, 2007 [DOI] [PubMed] [Google Scholar]
- 48.Rennenberg RJ, Kessels AG, Schurgers LJ, van Engelshoven JM, de Leeuw PW, Kroon AA: Vascular calcifications as a marker of increased cardiovascular risk: A meta-analysis. Vasc Health Risk Manag 5: 185–197, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Titapiccolo JI, Cerutti S, Garzotto F, Cruz D, Moissl U, Tetta C, Signorini MG, Ronco C, Ferrario M: Blood pressure variability and cardiovascular autonomic control during hemodialysis in peripheral vascular disease patients. Physiol Meas 33: 667–678, 2012 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.