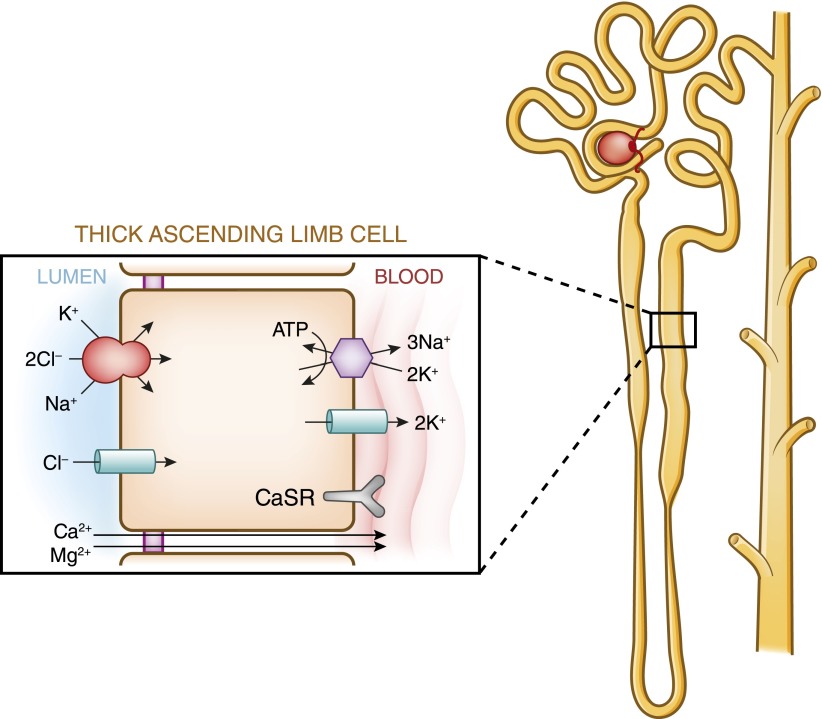
Figure 4.
Model of calcium and magnesium absorption by the thick ascending limb of Henle. Calcium absorption proceeds through both an active, transcellular pathway and by a passive paracellular pathway. Only transport pathways relevant to calcium absorption are shown. Basal absorption is passive and is driven by the ambient electrochemical gradient for calcium. The apical Na+-K+-2Cl− cotransporter and the renal outer medullary potassium K+ channel generate the “driving force” for paracellular cation transport. Calciotropic hormones, such as parathyroid hormone and calcitonin, stimulate active calcium absorption in cortical thick ascending limbs. Inhibition of Na-K-2Cl cotransport by loop diuretics or in Bartter’s syndrome decreases the transepithelial voltage, thus diminishing passive calcium absorption. In the model of magnesium absorption by thick ascending limb of Henle, 40%–70% of filtered magnesium is absorbed in the thick ascending limb by a paracellular pathway, mostly enhanced by lumen-positive transepithelial voltage. The apical Na-K-2Cl cotransporter mediates apical absorption of Na, K, and Cl. The apical renal outer medullary K channel mediates apical recycling of K back to the tubular lumen and generates lumen-positive voltage. Cl channel Kb mediates Cl exit through the basolateral membrane. Here Na,K-ATPase also mediates Na exit through the basolateral membrane and generates the Na gradient for Na absorption. The tight junction proteins claudin-16 and claudin-19 play a prominent role in magnesium absorption. The calcium-sensing receptor was also recently determined to regulate magnesium transport in this segment: upon stimulation, magnesium transport is decreased. CaSR, calcium-sensing receptor.