Fig. 4.
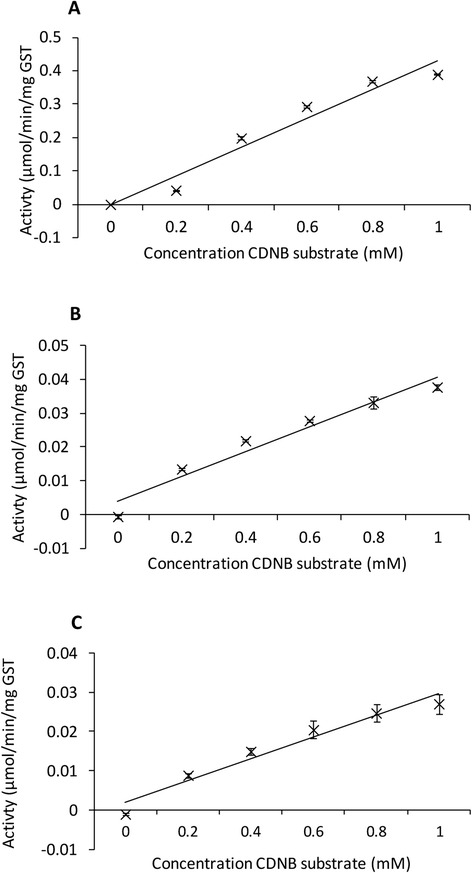
Enzymatic activity of three recombinant Dermanyssus gallinae GSTs proteins (rDeg-GSTs). The enzymatic activity of the purified rDeg-GST −1, −2 and −3 (panels a, b and c respectively) was determined over a 20 min period by measuring the increase in absorbance at 340 nm (A340nm) resulting from the GST-driven processing of the colorimetric 1-chloro-2,4-dinitrobenzene (CDNB) substrate present at a range of concentrations (0 to 1 mM). All assays were performed in triplicate with a constant concentration of 2 mM reduced glutathionine (GSH). The A340nm was adjusted for spontaneous substrate decay prior to calculating the mean specific activity (μmol/min/mg GST). The mean specific activity at the different CDNB concentrations is shown (± SEM, n = 3)
