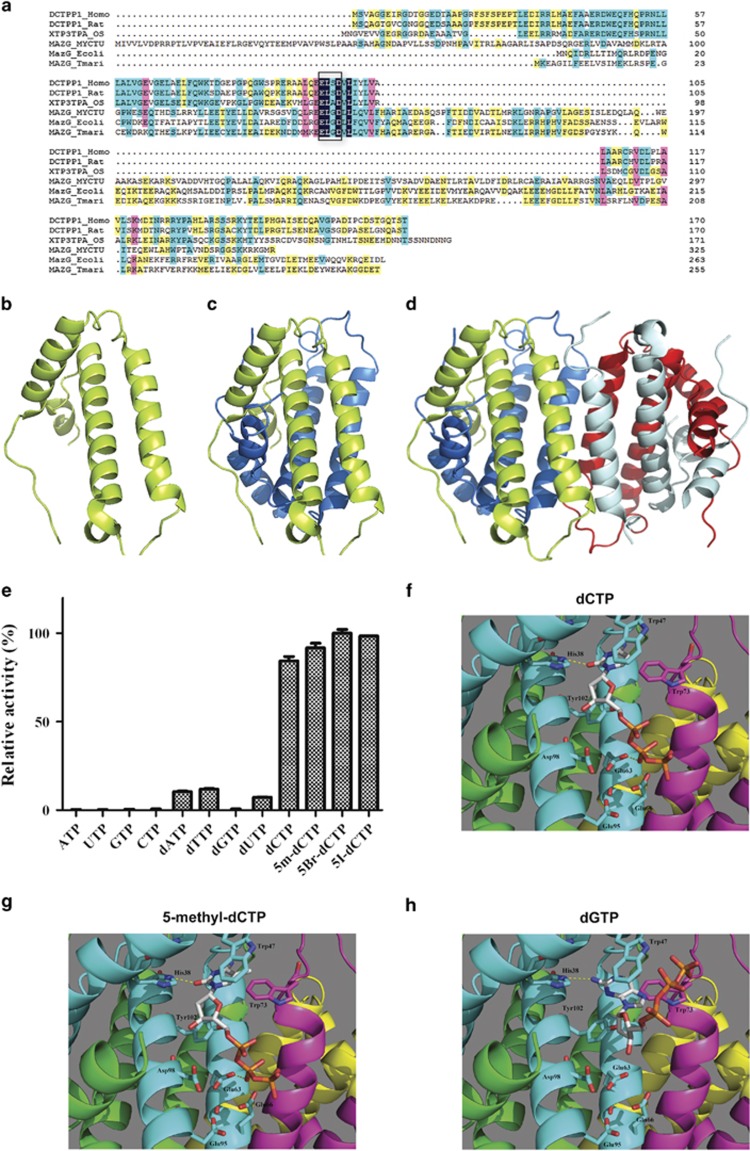
Figure 4.
DCTPP1 possesses MazG-like tetrameric domain through structure simulation and specifically catalyzes dCTP and its derivatives with structure complementarity. (a) DCTPP1 sequences from human, Rat, Oryza sativa and MazG proteins from E. coli, M.tb and Thermotoga maritima were aligned by DNAman software. The amino-acid residues with 100%, ⩾75%, ⩾50%, ⩾33% homolog were shown with indigo, pink, azure and yellow background, respectively. The EXXD motif was indicated by black frame. The 3-dimensional models of human DCTPP1 in monomeric (b), dimeric (c) and tetrameric (d) were generated by PyMol software. Protein monomer in tetrameric form was shown in limon, marine blue, pale cyan and red cartoons. The enzymatic activity of purified recombinant DCTPP1 was measured by using 200 μM (d)NTP substrates indicated (e). Binding modes of 5-methyl-dCTP (f), dCTP (g) and dGTP (h) to the human DCTPP1 tetramer were predicted by GLIDE software. Tetrameric DCTPP1 protein was composed by four monomers indicated in cyan, magenta, green and yellow cartoons, respectively. Oxygen atoms of dNTPs were shown in red and nitrogen atoms in blue. Carbon and phosphorus atoms of dNTPs were shown in white and orange, respectively. Crucial residues in the binding site were shown as stick and labeled. Hydrogen bonds are depicted in dotted line in yellow. Figures were generated by PyMOL Molecular Graphics System (DeLano Scientific, San Carlos, CA, USA).