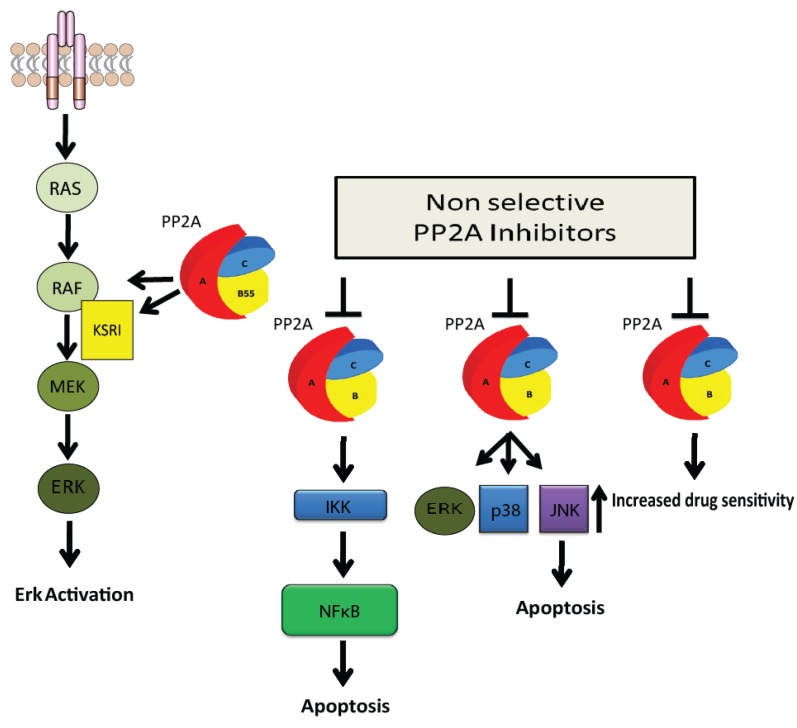
Figure 3.
PP2A as a promoter of carcinogenesis. The PP2A holoenzymes containing B55α and B55δ play a positive regulatory role in the MAP kinase pathway through direct dephosphorylation of RAF1 on Ser259. The PP2A holoenzyme containing B55α also dephophorylates KSR1 on Ser392 and RAF on Ser295 to activate ERK [31,32]. Inhibition of PP2A by Cantharidin promotes apoptosis in cancer cells by mediating the prolonged phosphorylation of I κ B kinase α (IKK) and activation of NFκB [145]. Cantharidin also induces apoptosis by blocking PP2A mediated activation of the MAP kinase pathway [81,145]. Inhibition of PP2A using okadaic acid increases the sensitivity of breast cancer cells to the drug lapatinib which has an anti-proliferative effect on cells [138].