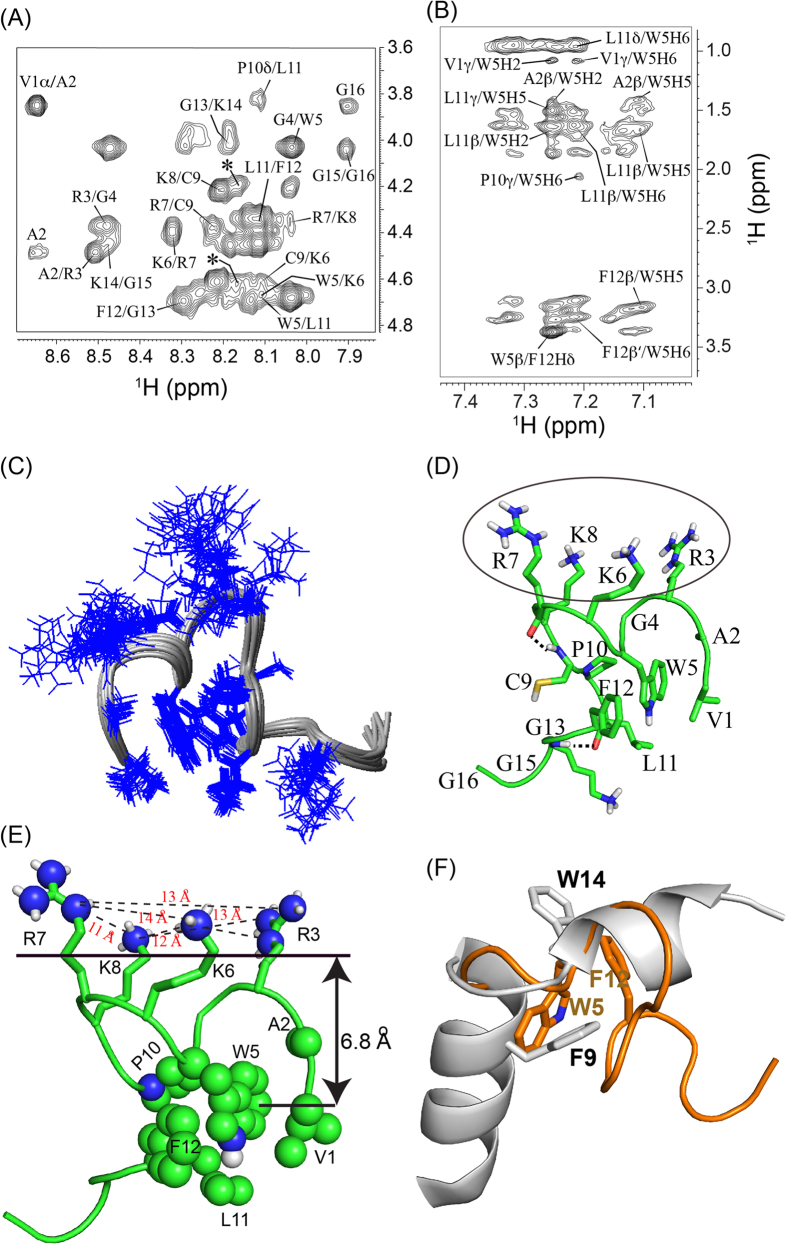
Figure 4. Structure of VG16KRKP in LPS.
Selected regions of two-dimensional 1H-1H trNOESY spectra of VG16KRKP in LPS showing (A) fingerprint region of CαH-NH resonances, and (B) long-range NOEs between aromatic ring protons and aliphatic side chain residues. Peaks, which are marked by the symbol * are unassigned due to the cis-trans configuration at Cys9-Pro10 bond. All experiments were performed on a Bruker Avance 500 MHz at 25 °C. (C) Side chain representation of twenty-ensemble VG16KRKP structures in LPS. (D) Cartoon representation of average structure of VG16KRKP conformations bound to LPS. The hydrogen bonds, which help in the stabilization of structures, are shown as black dotted lines. All the positive charges are facing one side, marked by circle. (E) Hydrophobic packing constituted by the residues Val1, Ala2, Trp5, Pro10, Leu11 and Phe12 are shown by space filling. Interestingly, the positive charge residues maintain a distance similar to the distance between two phosphate head groups of LPS (~12 Å). Depth of insertion study using fluorescence quencing experiments show that the position of Trp5 residue is ~6.8 Å from the center of the LPS bilayer, suggesting the Trp and othe residues of VG16KRKP, associated with Trp have strong van-der-Waals interaction with the acyl chain of LPS. (F) Superposition of DPC bound fusion domain of hemagglutinin (1IBN.pdb) (structure is stabilized by i, i + 5 residues) and LPS bound VG16KRKP (2 MWL.pdb) (structure is stabilized by i, i + 7 residues).