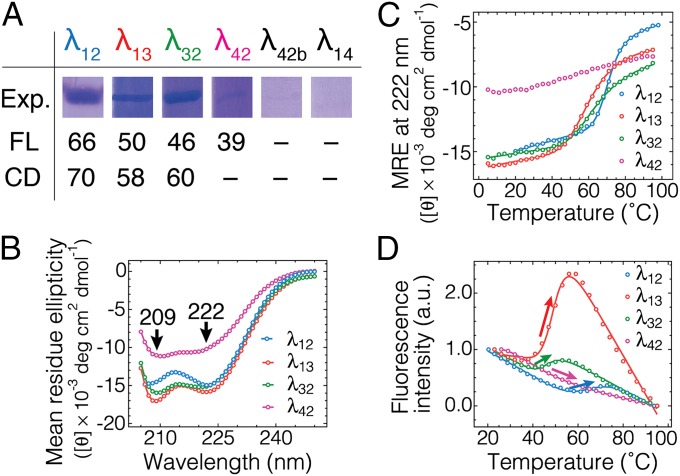
Fig. 2.
Expression and thermodynamic validation of engineered mutants. (A) Corresponding fractions of the protein purification gels for each mutant showing the relative expression yield. The midpoint denaturation temperatures in °C as determined by fluorescence intensity analysis (D; SI Appendix, Table S1) and circular dichroism (CD) at 222 nm as a proxy for α-helical content (C; SI Appendix, Table S1) are also shown. (B) CD spectra of four λ6–85 mutants collected at 23 °C. Negative peaks at 209 and 222 nm are characteristic of α-helical content. Data are in SI Appendix, Fig. S2 and Table S1. (C) Equilibrium temperature denaturation of four λ6–85 mutants monitored by CD at 222 nm as a proxy for α-content. Solid lines are two-state thermodynamic fits, which were used to determine the midpoint denaturation temperatures CD-Tm in A. λ42 was not fitted. (D) Equilibrium temperature denaturation of four λ6–85 mutants as monitored by fluorescence intensity integrated from 290 to 450 nm. The traces are normalized to begin at unity and end at zero. Solid lines are two-state fits, which were used to determine the midpoint denaturation temperatures FL-Tm in A. An increase in fluorescence intensity (arrows) is characteristic of the disruption of the Trp–Tyr interaction. Data are in SI Appendix, Fig. S3 and Table S1. Linear baselines are clearly a good approximation over our temperature range.