Abstract
Hepatocellular carcinoma (HCC) is among the most common cancer types and causes of cancer related mortality worldwide. Almost 50% of all HCC cases globally are attributable to chronic hepatitis B virus (HBV) infection. The incidence rates of HCC in untreated Asian subjects with HBV infection was estimated to be 0.2% in inactive carriers, 0.6% for those with chronic hepatitis without cirrhosis, and 3.7% for those with compensated cirrhosis. In Western populations, HCC incidences are reported to be 0.02% in inactive carriers, 0.3% in subjects with chronic hepatitis without cirrhosis, and 2.2% in subjects with compensated cirrhosis. Despite effective antiviral treatment options which are able to transform chronic hepatitis into an inactive carrier state, the risk of HCC cannot be fully ruled out to exclude those patients from surveillance. Newer nucleos(t)ide analogues (NAs) as entecavir and tenofovir are very potent in terms of sustained virological suppression which leads to improved liver histology. However, they do not have any influence on the cccDNA or integrated DNA of HBV in the liver. Nonetheless, viral replication is the only modifiable component among the established risk factors for HBV-related HCC with the current treatment options. In this review, it was aimed to summarize cumulative evidence behind the concept of prevention of HBV related HCC by NAs, and to discuss remaining obstacles to eliminate the risk of HCC.
Keywords: Hepatitis B virus, Hepatocellular carcinoma, Prevention, Nucleos(t)ide analogues, Risk factors
Core tip: After the introduction of potent nucleos(t)ide analogues with high genetic barrier to resistance, maintaining long-term virological suppression is achievable in almost all patients with chronic hepatitis B. The currently recommended first-line antiviral drugs, entecavir and tenofovir, can significantly reduce hepatocellular carcinoma (HCC) incidence, but the observed risk under efficient therapy is not zero in the long-term. There are established risk factors including age, gender, family history, low platelet levels, presence of cirrhosis or severity of liver disease, which should be incorporated into the clinical decision making to differentiate those patients under risk of developing HCC.
INTRODUCTION
Hepatocellular carcinoma (HCC) is the fifth most common cancer and third most common cause of cancer related mortality worldwide[1]. HCC virtually always develops within a background chronic liver disease. Globally, almost 50% of all HCC cases are attributable to chronic hepatitis B virus (HBV) infection[2]. HCC incidence is highly variable in different geographic locations and the distribution of the disease may be different even among ethnic groups living in the same country[1]. These differences are most probably due to geographical variations in the prevalence of hepatitis viruses. In Sub-Saharan Africa and Asia most cases of HCC develop in the presence of chronic hepatitis B (CHB) infection (up to 60%), however in Western countries only up to 20% of cases can be attributed to HBV infection[2]. Fortunately, after the introduction of universal vaccination programs throughout the world, the incidence of CHB infection was significantly decreased[3]. In the countries that have adopted the HBV vaccination program, a significant decrease in carrier rates as well as complications including HCC development have been experienced[4]. However, HBV infection is still a major health problem in most parts of the world and development of HCC is an important complication of chronic infection, even in inactive carrier patients[5]. In cirrhotic patients, surveillance for HCC increases the possibility of an earlier diagnosis that gives the patient a chance to undergo curative treatments[2]. However, current recommendations for screening of HCC in patients at risk is far from being satisfactory, and prognosis remains poor because treatment options are rather non-curative in advanced stages of the disease[6]. In this context, it is imperative to implement preventive strategies in patients with CHB infection. Despite effective antiviral treatment options which are able to transform chronic hepatitis into an inactive carrier state, the risk of HCC cannot be fully ruled out to exclude those patients from surveillance.
LITERATURE STUDY
In this review, it is sought to summarize the cumulative evidence on the role of antiviral therapy with NAs in the chemoprevention of HCC. Studies on prevention of HBV-related HCC were identified through electronic and manual search using online databases including MEDLINE and Web of Science. The relevant papers and conference proceedings in English language published from January, 2001 to January, 2015 were searched using following keywords: HCC, HBV, prevention, recurrence, curative treatment, resection, nucleos(t)ide analogue (NA), lamivudine, adefovir, telbivudine, entecavir, tenofovir. Selected studies were grouped according to one of the following topics: (1) NAs in chemoprevention of HCC; (2) HCC risk in patients who clear hepatitis B surface antigen (HBsAg); and (3) HCC recurrence risk in patients with HBV-related HCC after curative treatments. Both randomized-controlled and non-randomized studies were considered for inclusion. Uncontrolled studies were excluded unless an estimated HCC risk model was used to assess efficacy of NAs on HCC incidence. Reference lists of all papers, including reviews and meta-analyses, found during electronic search were checked manually to find relevant articles. The papers which were judged to be pertinent to the topic exceeded 100, and the number of included full-length articles were 51.
HBV AND THE RISK OF HCC
The association between HBV infection and development of HCC is well-established and has been demonstrated in several studies. In an early prospective controlled study from Asia, it was shown that the annual incidence of HCC was 0.5% in HBV-infected individuals; and the risk increased with age where annual incidence reached 1% at the age of 70[7]. HBV-infected subjects were found to be 100 times more likely to develop HCC compared to uninfected subjects. The incidence ratio for HCC was even higher as much as 2.5% per year in patients with known cirrhosis. In a recent study by Chen et al[5], a lesser but still substantial risk of HCC development was reported in an Asian cohort of patients with inactive HBV infection. In this study, the authors reported an annual incidence rate of 0.06% for HCC development with a relative risk of 4.4 compared to uninfected controls. In Western populations most studies are inadequate to drive conclusions and results are variable. This discrepancy between incidence rates among studies is probably due to the different patient settings (referral or population-based) and definitions for the HBV carrier state. Annual rates as high as 0.47% was reported in a study by Sherman et al[8], which can be explained by high prevalence (71%) of Asian background in this North American population. Yet, most studies reported that annual incidence of HBV-related HCC in Europe or North America seems to be less significant[9,10], and it is generally accepted to be around or less than 0.2%[11]. Therefore, it is not clear if surveillance is worthwhile or when is it cost-effective to start screening in Caucasian populations. HBV-infected patients with African ancestry seem to possess a higher risk of developing HCC, particularly at a younger age[12]. A database analysis from the United States reported higher incidence rates among Asians/Pacific Islanders, blacks, Native Americans/Alaska Natives, compared to people with European ancestry[13]. Although HCC may arise in the setting of inactive HBV infection, most patients with HBV who develop HCC have cirrhosis either long-standing or undiagnosed at the time of HCC diagnosis[14,15]. In a review of cohort studies[16], it was summarized that incidence rates of HCC in Asian subjects with HBV infection was estimated to be 0.2% in inactive carriers, 0.6% for those with chronic hepatitis without cirrhosis, and 3.7% for those with compensated cirrhosis. With an attention to inadequacy of studies in Western populations, they calculated an annual incidence rate of 0.02% in inactive carriers, 0.3% in subjects with chronic hepatitis without cirrhosis, and 2.2% in subjects with compensated cirrhosis.
There are a number of factors other than cirrhosis and ethnicity that have been reported to increase HCC risk among HBV carriers. Patient related factors include male gender, older age, high alcohol consumption and family history of HCC, and viral factors include duration of infection, higher viral replication, HBV genotype, and co-infection [hepatitis C, hepatitis D or human immunodeficiency virus (HIV)][14]. Recently, it was also shown that high levels of HBsAg titer (HBsAg ≥ 1000 IU/mL) is associated with increased risk of HCC in CHB patients with low viral load (HBVDNA < 2000 IU/mL)[17].
MECHANISMS OF HBV-ASSOCIATED HEPATOCARCINOGENESIS
An interaction of complex mechanisms and pathways contribute to the initiation of hepatocarcinogenesis. HBV infection is among the most important risk factors for development of HCC, and can influence carcinogenesis by multiple ways. Chronic inflammation, a driving factor in many types of cancers, is an important pathogenetic mechanism for development of HBV-related HCC. Chronic inflammation in the liver is characterized by sustained hepatic damage, damage-induced apoptosis, hepatocyte death/regeneration cycle, and tissue repair. In addition to continuous cycle of cell death and regeneration, constant activation of inflammatory signaling and increased cytokine production by innate and adaptive immune system contribute to generation of reactive oxygen and nitrogen species which can cause damage to important cellular components including cytoplasmic membrane lipids, intracellular proteins and DNA[18,19]. Genomic instability and alterations in epigenetic regulation of genes can cause inappropriate gene expression and enhanced proliferation of induced cells leading to neoplastic changes in susceptible individuals (Figure 1)[20]. Interestingly, induced cells may have clonal properties which were demonstrated in experimental models that show clonal hepatocyte repopulation is a major risk factor for HCC development[21,22]. In addition to non-specific hepatocarcinogenesis by chronic inflammation, HBV has unique virus-specific mechanisms involving the viral proteins HBx and preS/S, and the insertional mutagenesis with integration of HBV-DNA into the host genome that alters the expression of endogenous genes or induces chromosomal instability, and causes epigenetic changes including alterations in genomic methylation and regulation of microRNA expression (Figure 2)[23].
Figure 1.
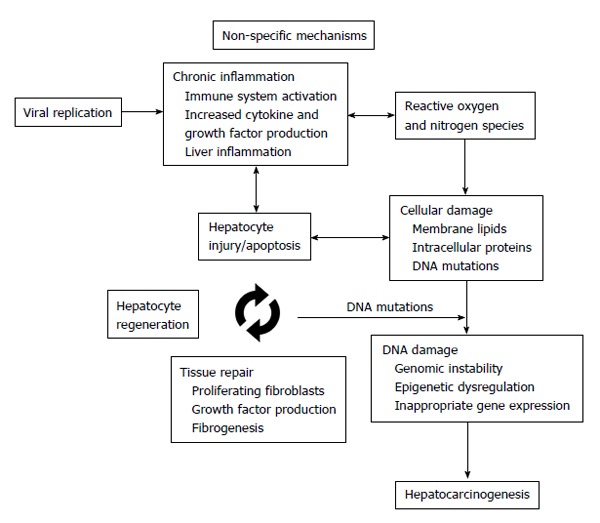
Chronic inflammation in the liver is characterized by sustained damage leading to hepatocyte death/regeneration and tissue repair cycle. Activation of inflammatory signaling with increased cytokine and growth factor production leads to oxidative stress which contributes to cellular damage. Cumulative damage to cellular structures, proteins and chromosomes alters genomic and epigenomic functions which can eventually induce hepatocarcinogenesis.
Figure 2.
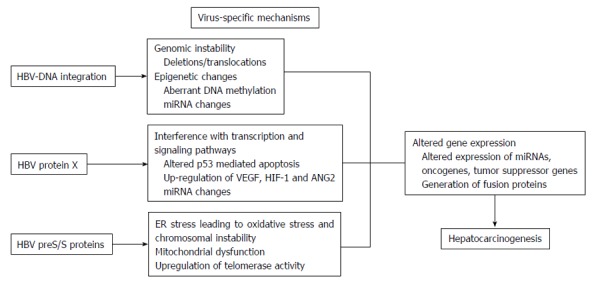
Hepatitis B virus has unique virus-specific mechanisms to induce carcinogenesis in the liver. The integration of hepatitis B virus (HBV)-DNA into the host genome alters DNA expression by gene and/or chromosomal deletions/translocations, and epigenetic changes (aberrant DNA methylation, miRNA changes). Viral protein HBV protein X may cause interference with transcription and signaling pathways leading to altered p53 mediated apoptosis and angiogenesis by up-regulation of vascular endothelial growth factor (VEGF), hypoxia inducible factor-1 (HIF-1) and angiopoietin-2 (ANG2). HBV preS/S proteins cause endoplasmic reticulum stress and mitochondrial dysfunction leading to oxidative stress.
NAs IN CHEMOPREVENTION OF HCC
The real key for certain prevention of HBV-related HCC is vaccination against the virus for newborns and people at risk. However in the absence of definitive curative treatment, there is a need for accurate risk estimation and modification in patients with chronic infection. Among the established risk factors for HBV-related HCC, patient or virus-related factors cannot be modified except viral replication. In a large prospective cohort by REVEAL-HBV study group, the relationship between HBV-DNA titer and HCC risk was demonstrated without any doubt[24,25]. Currently, there are five NAs approved for the treatment of patient with CHB: lamivudine, telbivudine, adefovir, entecavir and tenofovir disoproxil[26]. In the era of antiviral drugs with high barrier to resistance, we can suppress HBV-DNA in almost all patients receiving NAs, but the question remains if treatment prevents the development of HCC in every patient.
Lamivudine is a potent reverse transcriptase inhibitor which was originally developed for the treatment of patients with HIV infection. It is the first oral antiviral drug that was approved for treatment of patients with CHB. Lamivudine monotherapy in patients with CHB is associated with ALT normalization, HBV-DNA suppression, hepatitis B “e” antigen (HBeAg) seroconversion[27,28], and histological improvement (regression of necroinflammation and fibrosis) in the long-term[29]. The role of NAs to prevent HCC has been thoroughly investigated in multiple studies but the largest number of studies available considered only lamivudine. The studies which investigated effects of NAs in reduction of HCC risk in CHB are summarized in Table 1. In a study by Liaw et al[30], which evaluated long-term benefits of lamivudine monotherapy included 651 CHB patients with biopsy-proven advanced fibrosis or cirrhosis in a prospective randomized-placebo controlled setting. This pivotal study was terminated early due to significant beneficial effects seen in the treatment arm. Specifically, HCC development was observed in 3.9% of the patients in the treatment group and 7.4% of those in the placebo group with a hazard ratio of 0.49 (P = 0.047). In the same year, AISF (Italian Association for the Study of Liver Disease) Lamivudine Study Group investigated the effect of lamivudine treatment on the outcome of patients with HBeAg-negative CHB in a multicenter retrospective study[31]. They found that cirrhotic patients with maintained virological response were less likely to develop HCC and disease worsening. But, presence of cirrhosis and virological breakthrough were independently related to mortality and development of HCC. In another randomized-controlled trial by Chan et al[32], HBeAg-negative CHB patients were enrolled to lamivudine (100 mg/d) or placebo arms to investigate the efficacy of 2-year lamivudine treatment. Apparently, they did not find a risk reduction for HCC, but the study was not designed to answer this question and sample size was too small to get conclusions. Owing to undeniable beneficial effects of lamivudine therapy in CHB, subsequent randomized-controlled studies cannot be conducted to evaluate the influence of NAs. Thereafter, multiple case-control studies and prospective or retrospective cohort studies using historical controls were published. In a retrospective cohort study in Japan by Matsumoto et al[33], which included 377 treated patients (lamivudine 100 mg/d) vs 377 matched untreated controls showed a significant risk reduction for HCC in treated cohort (0.4% per year vs 2.5% per year, P < 0.001). Several other Asian cohort studies from China[34,35], South Korea[36-38] and India[39] also confirmed the protective effect of antiviral therapy with lamivudine against development of HCC. The evidence behind HCC chemoprevention with oral antivirals comes largely from Asian cohort studies; however data obtained in Caucasian populations also exists. In a European cohort study from Greece, Papatheodoridis et al[40] reported that lamivudine (with adefovir switch or add-on therapy when required) treatment significantly improved survival and reduced the risk of major events including HCC compared to interferon non-sustained responders and untreated controls.
Table 1.
Summary of controlled studies investigating the effect of nucleos(t)ide analogues on hepatocellular carcinoma risk among treated and untreated patients with chronic hepatitis B
| Ref. | Country/ region | Study type | Treatment | No. of patients (T/C) | Patient cohort | Follow-up (T/C), mean or median (yr) | Treatment outcome |
| Liaw et al[30] | Asia | RCT | Lamivudine (100 mg/d) | 436/215 | Advanced fibrosis or cirrhosis | 2.7/2.7 | Reduced HCC risk |
| Manolakopoulos et al[80] | Greece | Case-control | Lamivudine (100 mg/d) | 30/30 | Decompensated cirrhosis | 1.5/1.8 | No risk reduction |
| Matsumoto et al[33] | Japan | Retrospective cohort | Lamivudine (100 mg/d) | 377/377 | Chronic hepatitis B (any stage) | 2.7/5.3 | Reduced HCC risk |
| Papatheodoridis et al[40] | Greece | Retrospective cohort | Lamivudine (adefovir switch or add-on when required) | 201/195 | HBeAg-negative chronic hepatitis B | 3.8/6.1 | Better overall survival. Reduced risk of major events including HCC |
| Chan et al[32] | China | RCT | Lamivudine (100 mg/d) | 89/47 | HBeAg-negative chronic hepatitis B | 2.5/2.5 | No risk reduction |
| Yuen et al[34] | China | Prospective cohort | Lamivudine (25-100 mg/d) | 142/124 | HBeAg-positive chronic hepatitis B | 7.5/9.0 | Reduced cirrhosis/HCC risk |
| Lee et al[36] | South Korea | Retrospective cohort | Lamivudine (100 mg/d) | 589/589 | Chronic hepatitis B (any stage) | 2.9/5.3 | Reduced HCC risk |
| Ma et al[35] | China | Prospective cohort | Lamivudine (100 mg/d) | 41/176 | Cirrhosis | 3.16/NS | Reduced HCC risk |
| Das et al[39] | India | Case-control | Lamivudine or adefovir | 151/102 | Decompensated cirrhosis | 4.0/3.8 | Less HCC rate in treated group |
| Eun et al[37] | South Korea | Retrospective cohort | Lamivudine (100 mg/d) | 872/699 | Chronic hepatitis B (any stage) | 4.7/5.7 | Reduced HCC risk in cirrhotic patients with SVS |
| Kim et al[38] | South Korea | Retrospective cohort | Lamivudine and/or adefovir, or entecavir | 240/481 | Cirrhosis | 3.9/4.3 | Better overall survival. Reduced HCC risk (borderline significance) |
| Hosaka et al[46] | Japan | Retrospective cohort | Entecavir (0.5 mg/d) | 316/316 | Chronic hepatitis B (any stage) | 3.3/7.6 | Reduced HCC risk |
| Wong et al[47] | China | Retrospective cohort | Entecavir (0.5 mg/d) | 1446/424 | Chronic hepatitis B (any stage) | 3.0/9.5 | Reduced HCC risk in cirrhotic patients |
| Kumada et al[81] | Japan | Retrospective cohort | Lamivudine ± adefovir, entecavir | 117/117 | Chronic hepatitis B (any stage) | 12.3/11.6 | Reduced HCC risk |
| Sievert et al[49] | Reg. trial (abstract) | Prospective cohort | Tenofovir (300 mg/d) | 641 | Chronic hepatitis B (any stage) | 6 | Reduced HCC risk compared to estimated risk (REACH-B model) |
| Su et al[48] | Taiwan | Prospective cohort | Entecavir (0.5 mg/d) | 1123/503 | Cirrhosis (HBVDNA > 2000 IU/mL) | 3.6/6.8 | |
| Wu et al[44] | Taiwan | Retrospective nationwide cohort | Lamivudine, adefovir or entecavir | 21595/21595 | Chronic hepatitis B (any stage) | 3.5/5.2 | Reduced HCC risk |
| Gordon et al[82] | United States | Retrospective cohort | 94% received NAs, remaining received IFNs | 820/1851 | Chronic hepatitis B (any stage) | 5.2 | Reduced HCC risk |
| Coffin et al[83] | United States | Retrospective cohort | NAs | 322 | Chronic hepatitis B (any stage) | 3.2 | Reduced HCC risk compared to estimated risk (REACH-B model) |
NAs: Nucleos(t)ide analogues; HCC: Hepatocellular carcinoma; T: Treatment group; C: Control group; RCT: Randomized-controlled trial; HBeAg: Hepatitis B e antigen; SVS: Sustained virological suppression; IFNs: Interferons.
Despite these encouraging results of lamivudine monotherapy, the development of resistant strains of HBV have been the main problem since it was introduced into the clinical practice. An important finding of the study by Liaw et al[30] was that clinical deterioration defined as ≥ 2 increase in Child-Pugh score was significantly more frequent in patients who develop YMDD mutation under lamivudine treatment. In a large cohort study from South Korea, lamivudine treatment reduced the incidence of HCC both in patients with CHB and cirrhosis, yet the risk reduction was significant only for compensated cirrhotic patients and when the viral suppression was sustained[37]. Patients with virological breakthrough or suboptimal response during lamivudine therapy were shown to have an increased risk for HCC which was comparable to untreated controls. Kurokawa et al[41] evaluated 283 patients with CHB treated with lamivudine 100 mg/d in an uncontrolled cohort study. They found that maintained virological response, presence of cirrhosis, and age are independent risk factors for development of HCC in patients under lamivudine therapy.
In the era of NAs with high genetic barrier against resistance, entecavir and tenofovir are the only oral antiviral drugs recommended by major society treatment guidelines for CHB[26,42,43]. However, the evidence behind these novel oral antivirals regarding HCC risk reduction is scarce. In the largest retrospective nationwide CHB cohort from Taiwan[44], the investigators included 21595 matched patients in the treatment and control groups. Most of the patients were treated by lamivudine, yet 5748 patients received entecavir 0.5 mg/d. The treated cohort had a significantly lower 7-year incidence of HCC (7.32%; 95%CI: 6.77%-7.87%) than controls (22.7%; 95%CI: 22.1%-23.3%; P < 0.001). After adjusting for confounding factors, NA treatment was associated with a reduced risk of HCC, with an adjusted hazard ratio of 0.37 (95%CI: 0.34-0.39; P < 0.001). However, the authors did not report if there were any differences between lamivudine and entecavir treated patients regarding HCC incidence. In a randomized trial of 191 patients with decompensated cirrhosis, entecavir-treated patients tended to have a lower incidence of HCC than those treated with adefovir, but the risk reduction was not statistically significant (HR = 0.74, 95%CI: 0.46-1.18, P = 0.20)[45]. A recent study from Japan that compared the incidence of HCC in entecavir-treated patients and a matched historical cohort of untreated patients (316 vs 316 patients), the investigators found a significantly reduced risk of HCC in the treated group (5 years incidence rates were 3.7% and 13.7% for the treatment and control groups, respectively; P < 0.001)[46]. They also compared treatment effect between matched entecavir and lamivudine-treated patients without rescue therapy. It was reported that when the control group was taken as reference HCC risk reduction was more profound in entecavir-treated cirrhotic patients than it is for lamivudine-treated cirrhotic patients (P < 0.001 vs P = 0.019). Of note, this effect was seen in cirrhotic patients but not in non-cirrhotics. In a study from China by Wong et al[47], the cumulative probability of HCC between entecavir and untreated historical controls were comparable (P = 0.82). However, entecavir-treated patients with radiological cirrhosis had a significantly lower 5-year cumulative probability of HCC (13.8% vs 26.4%, P = 0.036) compared to the untreated patients with cirrhosis. This effect was more profound in cirrhotic patients with maintained virological suppression. On the other hand, entecavir-treated patients with cirrhosis who failed to achieve undetectable HBV-DNA had a comparable risk of HCC with the untreated patients. In a recent multicenter study from Taiwan[48], 1123 patients with HBV-related cirrhosis treated with entecavir were compared to 503 historical controls with HBV-related cirrhosis. All patients had baseline serum HBV-DNA level > 2000 IU/mL. Although treated patients were significantly older and had more advanced liver disease compared to historical controls, entecavir treatment was shown to be associated with an adjusted hazard ratio of 0.40 (95%CI: 0.27-0.60) in cirrhotic patients. After adjusting for age, the multivariate analysis showed that male gender, no treatment, lower albumin level and lower platelet count were independent risk factors associated with HCC development.
There are very few data regarding treatment effect of tenofovir on HCC incidence. One of them comes from post-hoc analysis of the registration trial of tenofovir, which was reported as an abstract. In this report by Sievert et al[49], investigators included 641 patients with CHB receiving open-label tenofovir therapy for 6 years and compared HCC incidence with the estimated risk calculated by REACH-B model[50]. They reported that 14 tenofovir-treated patients (6 of them were cirrhotic) developed HCC during the follow-up and the incidence of HCC decreased with a standardized incidence ratio of 0.45 (95%CI: 0.23-0.91) compared to the estimated risk. Despite the low number of HCC cases in this study, they concluded by emphasizing continued surveillance for CHB patients receiving long term oral antiviral treatment.
There are several meta-analyses or systematic reviews that confirmed the beneficial effects of NAs in the prevention of HCC in CHB. In a systematic review by Papatheodoridis et al[51], 21 studies were reviewed and 3 of them which were of high quality and included untreated controls. In the pooled analysis of 3 studies, HCC was detected significantly less frequently in treated than in untreated patients (2.8% vs 6.4%, respectively, P = 0.003). Interestingly, they found that incidence of HCC was significantly higher in untreated patients even when compared to treated patients with virological breakthroughs or no response. Similarly, other meta-analyses confirmed these results regarding the influence of NAs on HCC risk (Table 2)[52-54].
Table 2.
Systematic review and meta-analyses investigating hepatocellular carcinoma risk reduction in patients receiving nucleos(t)ide analogues vs untreated controls
| Ref. | No. of studies | No. of patients (T/C) | OR (95%CI) | P |
| Papatheodoridis et al[51] | 3 | 1313 (779/534) | 0.43 (0.25-0.74) | 0.002 |
| Zhang et al[52] | 6 | 3644 (2035/1609) | 0.26 (0.15-0.47) | < 0.00001 |
| Singal et al[53] | 6 | 6877 (3306/3571) | 0.48 (0.38-0.61) | < 0.00001 |
| Sung et al[54] | 5 | 2289 (1267/1022) | 0.22 (0.10-0.50) | 0.0003 |
T: Treatment group; C: Control group.
Although it is evident to say that antiviral therapy decrease HBV-related HCC incidence when compared with the natural course of the disease, there are sufficient data showing that antiviral therapy does not eliminate the HCC risk completely, even in non-cirrhotic patients with sustained virological suppression. Papatheodoridis et al[55] included 818 patients with HBeAg-negative CHB treated with NAs in a retrospective study investigating HCC incidence. All patients were treated with NAs starting with lamivudine monotherapy, and during a median follow-up of 4.7 years 49 patients (6%) eventually developed HCC. The study demonstrated a trend for lower cumulative HCC incidence in CHB patients with virological on-therapy remission (P = 0.076), which was defined as maintained undetectable HBV-DNA (< 200 IU/mL). However, virological remission did not significantly influence the incidence of HCC in patients with cirrhosis (0.327). Moreover, multivariate analysis revealed that age, gender and cirrhosis were independently associated with HCC risk regardless of virological remission. In a recent study by Arends et al[56], 14 of 744 patients (42% Caucasian ethnicity) with CHB developed HCC during a median follow-up of 167 wk. Nine (64%) patients among them had cirrhosis at baseline, and 12 patients developed HCC even after achieving virological response (HBV-DNA < 80 IU/mL). The 5-year cumulative incidence rate of HCC was reported to be low for non-cirrhotic patients, yet it was significantly higher for cirrhotic patients (2.1% vs 10.9%, respectively, P < 0.001).
Several studies investigated independent risk factors associated with HCC development in patients receiving NAs. The factors commonly attributed to HCC risk in patients receiving NAs were age, male gender, duration of disease, presence of cirrhosis and no virological response[41,51,55,57-59]. Recently in a large European retrospective multicenter cohort of 1666 patients with CHB (all Caucasian) treated with entecavir or tenofovir, 71 patients (4.3%) developed HCC within a median follow-up duration of 39 mo (range, 8-140 mo)[60]. The importance of this study is that there was little information available regarding the risk factors of HCC in Caucasian patients treated with novel antiviral drugs. The authors reported an annual incidence rate of 1.37 (95%CI: 1.09-1.73) per 100 patient. The cumulative probability of developing HCC reached 8.7% after 5 years of antiviral therapy. In multivariate Cox regression analysis they found age, male gender, low platelet levels (< 100000/mm3), and liver disease severity were independently associated with subsequent development of HCC. Of note, they did not find a significant association between virological remission under treatment and risk of HCC development, which was contrary to previous studies. However, this result is probably related to the high virological remission rate (92%) under antiviral treatment. A recent study from Turkey; which investigated the risk of HCC development in 641 CHB patients on-therapy; showed that cirrhosis, NAs with low-genetic barrier against resistance, and development of resistance or virological breakthrough were independent predictors of HCC development[61].
HCC RISK IN PATIENTS WHO CLEAR HBSAG
Whether it is spontaneous or therapy-induced, HBsAg clearance with or without anti-HBs seroconversion is considered highly beneficial. Although HBsAg loss or seroconversion has been the ultimate goal in the treatment of CHB, it is rarely achievable with the available NA options which have no influence on intrahepatic cccDNA of HBV. Nonetheless, it is still the holy grail of a treatment course which can be achieved by only a small group of patients, even in the long-term. However, until recently data was insufficient to determine if it is reasonable to exclude those patients who achieve HBsAg loss or seroconversion from routine clinical follow-up. Although there is evidence showing that disease progression may still occur after HBsAg loss[62], the common practice have been continuing follow-up and HCC screening only in those with cirrhosis. This seems to be an evidence-based approach, because cirrhosis is the major risk factor for HCC and should be considered a premalignant condition even after HBsAg loss or seroconversion[63]. In a recent study by Simonetti et al[64], 1271 Alaskan native patients with CHB were included in a prospective population-based cohort study, and 158 patients achieved HBsAg loss during a mean follow-up duration of 19.6 years. The authors reported that 6 patients (2 of them were cirrhotic) developed HCC during a mean follow-up of 7.3 years after HBsAg clearance. Although the HCC incidence after HBsAg clearance was reported to be significantly lower than the rate in those who remained seropositive, the risk was still high enough to justify continuum of periodic follow-up visits with HCC surveillance.
HCC RECURRENCE RISK IN PATIENTS WITH HBV-RELATED HCC AFTER CURATIVE TREATMENTS
Despite advances in surgical techniques, survival after curative resection for HBV-related HCC remains dissatisfactory with recurrence rates of more than 50%[65]. There is no proven adjuvant chemotherapeutic regimen that can reduce recurrence risk or improve patient survival after curative resection. However, the above mentioned body of data provides solid evidence that antiviral therapy in patients with CHB or cirrhosis reduces the risk of HCC development, leading to the notion that antiviral therapy might prevent recurrence and improve survival after curative therapy. There is convincing evidence that persistent high viremia is associated with increased risk of HCC recurrence in CHB patients who underwent resection for HBV-related HCC[66,67]. Therefore, the aim has been focused on suppression of persistent HBV replication, which occurs in almost all patients after liver resection and can severely reduce liver function and survival[68]. Initial studies with small sample sizes did not find any significant effect of NAs to prevent HCC recurrence[69-71]. In the first study that demonstrated a beneficial effect, Kubo et al[72] found a lower 5-year disease-free survival rate after surgery in the lamivudine-treated group than control group. Subsequent studies provided conflicting results most probably due to the insufficient number of patients included, treatment heterogeneity (resection, local ablation, chemoembolization), and short duration of follow-up after resection (Table 3). For example, Chan et al[73] reported a significantly higher 5-year tumor-free survival rates in the treatment group (42 patients, lamivudine 100 mg/d or entecavir 0.5 mg/d) than in the control group (94 patients) (51.4% vs 33.8%, respectively, P = 0.05). In contrast, Li et al[74] demonstrated a higher but insignificant 1-year tumor free survival after curative hepatectomy in treated (Lamivudine ± adefovir) vs control groups (23.3% vs 8.3%, respectively, P = 0.072). Yet, the duration of follow-up after resection was too short to get conclusions. A larger study with a longer duration of follow-up in a retrospective nationwide cohort by Wu et al[75] demonstrated a significantly reduced HCC recurrence rate in patients receiving NAs after liver resection. Two recently published randomized-controlled trials and several meta-analyses (Table 4) also confirmed these results which were obtained from retrospective cohort studies[65,76-79].
Table 3.
Summary of controlled studies investigating the efficacy of nucleos(t)ide analogues in prevention of hepatocellular carcinoma recurrence after curative treatments
| Ref. | Country | Study type | Antiviral treatment | No. of patients (T/C) | HCC treatment | Follow-up (T/C), mean/median (yr) | Treatment outcome |
| Piao et al[69] | Japan | Retrospective cohort | Lamivudine (100 mg/d) | 30/40 | Resection or ablation/TACE | Not specified | No risk reduction for HCC recurrence |
| Better overall survival | |||||||
| Shuqun et al[70] | China | Retrospective cohort | Lamivudine (100 mg/d) + thymosin α1 | 17/16 | Resection | 1-3 | Reduced HCC recurrence (NS) |
| Kuzuya et al[71] | Japan | Retrospective cohort | Lamivudine (100 mg/d) | 16/33 | Resection or RFA | 3.2/2.7 | No risk reduction |
| Kubo et al[72] | Japan | Retrospective cohort | Lamivudine (100 mg/d) | 14/10 | Resection | 2.1 | Higher tumor-free survival |
| Yoshida et al[84] | Japan | Retrospective cohort | Lamivudine (100 mg/d) | 33/71 | RFA | 2.8/3.9 | No risk reduction |
| Hung et al[85] | China | Retrospective cohort | Lamivudine (100 mg/d) | 10/62 | Resection | 1.6 | Reduced HCC recurrence |
| Koda et al[86] | Japan | Retrospective cohort | Lamivudine or entecavir | 30/20 | Resection or ablation/TAE | 2.4/3.0 | No risk reduction for HCC recurrence |
| Better overall survival | |||||||
| Chuma et al[87] | Japan | Retrospective cohort | Lamivudine (100 mg/d) | 39/64 | Resection or RFA | 2.9/4.4 | Reduced HCC recurrence |
| Li et al[74] | China | Prospective cohort | Lamivudine ± adefovir | 43/36 | Resection | 1.0 | No risk reduction |
| Chan et al[73] | China | Retrospective cohort | Lamivudine or entecavir | 42/94 | Resection | Not specified | Reduced HCC recurrence |
| Urata et al[88] | Japan | Retrospective cohort | Not specified | 46/242 | Resection | 3.1 | Tumor-free survival is better vs patients with high viral load |
| Yang et al[89] | China | Prospective cohort | Lamivudine, adefovir or entecavir | 142/188 | Resection | 4.0 | Reduced HCC recurrence |
| Wu et al[75] | Taiwan | Retrospective nationwide cohort | Nucleoside analogue(s) | 518/4051 | Resection | 2.6/2.2 | Reduced HCC recurrence |
| Huang et al[90] | China | Prospective cohort | Lamivudine, adefovir or entecavir | 865/175 | Resection (HBVDNA > 2000 IU/mL) | 3.5 | Better disease-free survival (borderline significance) |
| Better overall survival | |||||||
| Huang et al[68] | China | Retrospective cohort | Lamivudine, adefovir or entecavir | 150/1459 | Resection (HBVDNA < 2000 IU/mL) | 2.9-3.3 | Better disease-free survival |
| Ke et al[91] | China | Retrospective cohort | Lamivudine (100 mg/d) | 141/337 | Resection | 2.0/1.9 | No risk reduction for HCC recurrence |
| Better overall survival | |||||||
| Su et al[92] | Taiwan | Retrospective cohort | Lamivudine, entecavir or pegylated interferon | 62/271 | Resection | 3.8 | Reduced HCC recurrence |
| Better overall survival | |||||||
| Yin et al[76] | China | 2 cohorts (RCT and NRC) | Lamivudine1 (100 mg/d) | RCT: 81/82; NRC: 215/402 | Resection | RCT: 3.3; NRC: 1.98 | Reduced HCC recurrence and better overall survival in both cohorts |
| Yeh et al[93] | Taiwan | Retrospective cohort | Entecavir, lamivudine, telbivudine, or combination | 490/3369 | Resection, RFA, PEI | 3.3/3.3 | No benefits for HCC progression or overall survival |
| Zhang et al[94] | China | Retrospective cohort | Entecavir (0.5 mg/d) | 40/47 | Resection | 2.6 | Reduced recurrence if HCC ≤ 3 cm |
| Hann et al[95] | United States | Retrospective cohort | NAs | 16/9 | Resection, RFA, PEI, TACE | 5.0 | Reduced HCC recurrence |
| Better overall survival | |||||||
| Nishikawa et al[96] | Japan | Retrospective cohort | Lamivudine, adefovir or entecavir | 99/32 | Resection, RFA, PEI | 3.5/4.0 | No risk reduction for HCC recurrence |
| Better overall survival | |||||||
| Huang et al[77] | China | RCT | Adefovir (10 mg/d) | 100/100 | Resection | 5.0 | Reduced HCC recurrence |
| Switch to entecavir (18 patients) | Better overall survival | ||||||
| Chong et al[97] | China | Retrospective-prospective cohort | Nucleoside analog(s) | 254/150 | Resection | 3.3/3.6 | No risk reduction for HCC recurrence |
| Better overall survival |
Adefovir ± lamivudine or entecavir for drug resistance;
13 patients with high viral load, 11 patients with low viral load. HCC: Hepatocellular carcinoma; T: Treatment group; C: Control group; NS: Not significant; TACE: Transarterial chemoembolization; TAE: Transarterial embolization; RFA: Radiofrequency ablation; PEI: Percutaneous ethanol injection; NAs: Nucleos(t)ide analogues; RCT: Randomized-controlled trial; NRC: Non-randomized cohort.
Table 4.
Summary of meta-analyses which investigated preventive effect of nucleos(t)ide analogues on hepatocellular carcinoma recurrence in patients who underwent curative treatments
| Ref. | No. of studies | No. of patients (T/C) | OR (95%CI) | P |
| Miao et al[98] | ||||
| 1 yr recurrence | 2 | 119 (46/73) | 0.59 (0.24-1.43) | 0.24 |
| 2 yr recurrence | 2 | 119 (46/73) | 0.82 (0.34-1.74) | 0.60 |
| Wong et al[65] | 9 | 555 (204/347) | 0.59 (0.35-0.97) | 0.04 |
| Sun et al[78] | 13 | 6350 (1227/5123) | 0.66 (0.54-0.80) | < 0.0001 |
| Zhou et al[79] | 8 | 6127 (NS) | 0.69 (0.59-0.80) | < 0.00001 |
NS: Not significant; T: Treatment group; C: Control group.
CONCLUSION
In terms of HBV and hepatocarcinogenesis, it is important to emphasize that unlike other chronic liver diseases, HCC is not always seen on a background of advanced fibrosis or cirrhosis in CHB patients. Many studies confirmed the concept of suppression of HBV replication for chemoprevention of HCC in either primary or secondary prevention settings. Many of those studies are not perfect, but cumulative evidence shows an undeniable beneficial effect of NAs. Although the effect of modern NAs cannot be assessed in prospective-controlled trials which include placebo-treated or untreated patients in the control groups, there is no doubt that entecavir or tenofovir can provide greater and long-term virological suppression leading to reduced HCC incidence and better patient survival. Regardless of the antiviral treatment, several risk factors including but not limited to old age, male gender, longer duration of the disease, inadequate virological suppression and presence of cirrhosis are clearly associated with the ongoing risk of HCC development. Fortunately, inadequate virological suppression is not an issue with the newer antivirals, if patients are adherent with their medications. Recent evidence also shows that treatment with NAs cannot completely wipe out the risk of HCC, even in patients without risk factors or patients who clear HBsAg. Therefore, future studies should focus on differentiating patients who remain under risk despite effective antiviral therapy and providing cost-effective surveillance strategies in those patients under risk of HCC.
Footnotes
P- Reviewer: Galvao FHF, Grassi A, Solinas A S- Editor: Tian YL L- Editor: A E- Editor: Liu SQ
Conflict-of-interest statement: None.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: February 2, 2015
First decision: April 10, 2015
Article in press: June 19, 2015
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.European Association For The Study Of The Liver; European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908–943. doi: 10.1016/j.jhep.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 3.Ott JJ, Stevens GA, Groeger J, Wiersma ST. Global epidemiology of hepatitis B virus infection: new estimates of age-specific HBsAg seroprevalence and endemicity. Vaccine. 2012;30:2212–2219. doi: 10.1016/j.vaccine.2011.12.116. [DOI] [PubMed] [Google Scholar]
- 4.Chang MH, You SL, Chen CJ, Liu CJ, Lee CM, Lin SM, Chu HC, Wu TC, Yang SS, Kuo HS, et al. Decreased incidence of hepatocellular carcinoma in hepatitis B vaccinees: a 20-year follow-up study. J Natl Cancer Inst. 2009;101:1348–1355. doi: 10.1093/jnci/djp288. [DOI] [PubMed] [Google Scholar]
- 5.Chen JD, Yang HI, Iloeje UH, You SL, Lu SN, Wang LY, Su J, Sun CA, Liaw YF, Chen CJ. Carriers of inactive hepatitis B virus are still at risk for hepatocellular carcinoma and liver-related death. Gastroenterology. 2010;138:1747–1754. doi: 10.1053/j.gastro.2010.01.042. [DOI] [PubMed] [Google Scholar]
- 6.El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365:1118–1127. doi: 10.1056/NEJMra1001683. [DOI] [PubMed] [Google Scholar]
- 7.Beasley RP, Hwang LY, Lin CC, Chien CS. Hepatocellular carcinoma and hepatitis B virus. A prospective study of 22 707 men in Taiwan. Lancet. 1981;2:1129–1133. doi: 10.1016/s0140-6736(81)90585-7. [DOI] [PubMed] [Google Scholar]
- 8.Sherman M, Peltekian KM, Lee C. Screening for hepatocellular carcinoma in chronic carriers of hepatitis B virus: incidence and prevalence of hepatocellular carcinoma in a North American urban population. Hepatology. 1995;22:432–438. [PubMed] [Google Scholar]
- 9.Crook PD, Jones ME, Hall AJ. Mortality of hepatitis B surface antigen-positive blood donors in England and Wales. Int J Epidemiol. 2003;32:118–124. doi: 10.1093/ije/dyg039. [DOI] [PubMed] [Google Scholar]
- 10.Villeneuve JP, Desrochers M, Infante-Rivard C, Willems B, Raymond G, Bourcier M, Côté J, Richer G. A long-term follow-up study of asymptomatic hepatitis B surface antigen-positive carriers in Montreal. Gastroenterology. 1994;106:1000–1005. doi: 10.1016/0016-5085(94)90760-9. [DOI] [PubMed] [Google Scholar]
- 11.Kew MC. Epidemiology of chronic hepatitis B virus infection, hepatocellular carcinoma, and hepatitis B virus-induced hepatocellular carcinoma. Pathol Biol (Paris) 2010;58:273–277. doi: 10.1016/j.patbio.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 12.Kew MC, Macerollo P. Effect of age on the etiologic role of the hepatitis B virus in hepatocellular carcinoma in blacks. Gastroenterology. 1988;94:439–442. doi: 10.1016/0016-5085(88)90434-9. [DOI] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention (CDC) Hepatocellular carcinoma - United States, 2001-2006. MMWR Morb Mortal Wkly Rep. 2010;59:517–520. [PubMed] [Google Scholar]
- 14.El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012;142:1264–1273.e1. doi: 10.1053/j.gastro.2011.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang JD, Kim WR, Coelho R, Mettler TA, Benson JT, Sanderson SO, Therneau TM, Kim B, Roberts LR. Cirrhosis is present in most patients with hepatitis B and hepatocellular carcinoma. Clin Gastroenterol Hepatol. 2011;9:64–70. doi: 10.1016/j.cgh.2010.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fattovich G, Bortolotti F, Donato F. Natural history of chronic hepatitis B: special emphasis on disease progression and prognostic factors. J Hepatol. 2008;48:335–352. doi: 10.1016/j.jhep.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 17.Tseng TC, Liu CJ, Yang HC, Su TH, Wang CC, Chen CL, Kuo SF, Liu CH, Chen PJ, Chen DS, et al. High levels of hepatitis B surface antigen increase risk of hepatocellular carcinoma in patients with low HBV load. Gastroenterology. 2012;142:1140–1149.e3; quiz e13-14. doi: 10.1053/j.gastro.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 18.Lu H, Ouyang W, Huang C. Inflammation, a key event in cancer development. Mol Cancer Res. 2006;4:221–233. doi: 10.1158/1541-7786.MCR-05-0261. [DOI] [PubMed] [Google Scholar]
- 19.Kundu JK, Surh YJ. Inflammation: gearing the journey to cancer. Mutat Res. 2008;659:15–30. doi: 10.1016/j.mrrev.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 20.Wu Y, Antony S, Meitzler JL, Doroshow JH. Molecular mechanisms underlying chronic inflammation-associated cancers. Cancer Lett. 2014;345:164–173. doi: 10.1016/j.canlet.2013.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mason WS, Jilbert AR, Summers J. Clonal expansion of hepatocytes during chronic woodchuck hepatitis virus infection. Proc Natl Acad Sci USA. 2005;102:1139–1144. doi: 10.1073/pnas.0409332102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marongiu F, Doratiotto S, Montisci S, Pani P, Laconi E. Liver repopulation and carcinogenesis: two sides of the same coin? Am J Pathol. 2008;172:857–864. doi: 10.2353/ajpath.2008.070910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tarocchi M, Polvani S, Marroncini G, Galli A. Molecular mechanism of hepatitis B virus-induced hepatocarcinogenesis. World J Gastroenterol. 2014;20:11630–11640. doi: 10.3748/wjg.v20.i33.11630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen CJ, Yang HI, Su J, Jen CL, You SL, Lu SN, Huang GT, Iloeje UH. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA. 2006;295:65–73. doi: 10.1001/jama.295.1.65. [DOI] [PubMed] [Google Scholar]
- 25.Chen CF, Lee WC, Yang HI, Chang HC, Jen CL, Iloeje UH, Su J, Hsiao CK, Wang LY, You SL, et al. Changes in serum levels of HBV DNA and alanine aminotransferase determine risk for hepatocellular carcinoma. Gastroenterology. 2011;141:1240–1248, 1248.e1-2. doi: 10.1053/j.gastro.2011.06.036. [DOI] [PubMed] [Google Scholar]
- 26.European Association For The Study Of The Liver. EASL clinical practice guidelines: Management of chronic hepatitis B virus infection. J Hepatol. 2012;57:167–185. doi: 10.1016/j.jhep.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 27.Dienstag JL, Schiff ER, Wright TL, Perrillo RP, Hann HW, Goodman Z, Crowther L, Condreay LD, Woessner M, Rubin M, et al. Lamivudine as initial treatment for chronic hepatitis B in the United States. N Engl J Med. 1999;341:1256–1263. doi: 10.1056/NEJM199910213411702. [DOI] [PubMed] [Google Scholar]
- 28.Lai CL, Chien RN, Leung NW, Chang TT, Guan R, Tai DI, Ng KY, Wu PC, Dent JC, Barber J, et al. A one-year trial of lamivudine for chronic hepatitis B. Asia Hepatitis Lamivudine Study Group. N Engl J Med. 1998;339:61–68. doi: 10.1056/NEJM199807093390201. [DOI] [PubMed] [Google Scholar]
- 29.Dienstag JL, Goldin RD, Heathcote EJ, Hann HW, Woessner M, Stephenson SL, Gardner S, Gray DF, Schiff ER. Histological outcome during long-term lamivudine therapy. Gastroenterology. 2003;124:105–117. doi: 10.1053/gast.2003.50013. [DOI] [PubMed] [Google Scholar]
- 30.Liaw YF, Sung JJ, Chow WC, Farrell G, Lee CZ, Yuen H, Tanwandee T, Tao QM, Shue K, Keene ON, Dixon JS, Gray DF, Sabbat J. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med. 2004;351:1521–1531. doi: 10.1056/NEJMoa033364. [DOI] [PubMed] [Google Scholar]
- 31.Di Marco V, Marzano A, Lampertico P, Andreone P, Santantonio T, Almasio PL, Rizzetto M, Craxì A. Clinical outcome of HBeAg-negative chronic hepatitis B in relation to virological response to lamivudine. Hepatology. 2004;40:883–891. doi: 10.1002/hep.20381. [DOI] [PubMed] [Google Scholar]
- 32.Chan HL, Wang H, Niu J, Chim AM, Sung JJ. Two-year lamivudine treatment for hepatitis B e antigen-negative chronic hepatitis B: a double-blind, placebo-controlled trial. Antivir Ther. 2007;12:345–353. [PubMed] [Google Scholar]
- 33.Matsumoto A, Tanaka E, Rokuhara A, Kiyosawa K, Kumada H, Omata M, Okita K, Hayashi N, Okanoue T, Iino S, et al. Efficacy of lamivudine for preventing hepatocellular carcinoma in chronic hepatitis B: A multicenter retrospective study of 2795 patients. Hepatol Res. 2005;32:173–184. doi: 10.1016/j.hepres.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 34.Yuen MF, Seto WK, Chow DH, Tsui K, Wong DK, Ngai VW, Wong BC, Fung J, Yuen JC, Lai CL. Long-term lamivudine therapy reduces the risk of long-term complications of chronic hepatitis B infection even in patients without advanced disease. Antivir Ther. 2007;12:1295–1303. [PubMed] [Google Scholar]
- 35.Ma H, Wei L, Guo F, Zhu S, Sun Y, Wang H. Clinical features and survival in Chinese patients with hepatitis B e antigen-negative hepatitis B virus-related cirrhosis. J Gastroenterol Hepatol. 2008;23:1250–1258. doi: 10.1111/j.1440-1746.2008.05499.x. [DOI] [PubMed] [Google Scholar]
- 36.Lee HJ, Eun R, Jang BI, Kim TN. Prevention by Lamivudine of hepatocellular carcinoma in patients infected with hepatitis B virus. Gut Liver. 2007;1:151–158. doi: 10.5009/gnl.2007.1.2.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eun JR, Lee HJ, Kim TN, Lee KS. Risk assessment for the development of hepatocellular carcinoma: according to on-treatment viral response during long-term lamivudine therapy in hepatitis B virus-related liver disease. J Hepatol. 2010;53:118–125. doi: 10.1016/j.jhep.2010.02.026. [DOI] [PubMed] [Google Scholar]
- 38.Kim CH, Um SH, Seo YS, Jung JY, Kim JD, Yim HJ, Keum B, Kim YS, Jeen YT, Lee HS, et al. Prognosis of hepatitis B-related liver cirrhosis in the era of oral nucleos(t)ide analog antiviral agents. J Gastroenterol Hepatol. 2012;27:1589–1595. doi: 10.1111/j.1440-1746.2012.07167.x. [DOI] [PubMed] [Google Scholar]
- 39.Das K, Das K, Datta S, Pal S, Hembram JR, Dhali GK, Santra A, Chowdhury A. Course of disease and survival after onset of decompensation in hepatitis B virus-related cirrhosis. Liver Int. 2010;30:1033–1042. doi: 10.1111/j.1478-3231.2010.02255.x. [DOI] [PubMed] [Google Scholar]
- 40.Papatheodoridis GV, Dimou E, Dimakopoulos K, Manolakopoulos S, Rapti I, Kitis G, Tzourmakliotis D, Manesis E, Hadziyannis SJ. Outcome of hepatitis B e antigen-negative chronic hepatitis B on long-term nucleos(t)ide analog therapy starting with lamivudine. Hepatology. 2005;42:121–129. doi: 10.1002/hep.20760. [DOI] [PubMed] [Google Scholar]
- 41.Kurokawa M, Hiramatsu N, Oze T, Yakushijin T, Miyazaki M, Hosui A, Miyagi T, Yoshida Y, Ishida H, Tatsumi T, et al. Long-term effect of lamivudine treatment on the incidence of hepatocellular carcinoma in patients with hepatitis B virus infection. J Gastroenterol. 2012;47:577–585. doi: 10.1007/s00535-011-0522-7. [DOI] [PubMed] [Google Scholar]
- 42.Liaw YF, Kao JH, Piratvisuth T, Chan HLY, Chien RN, Liu CJ, Gane E, Locarnini S, Lim SG, Han KH, et al. Asian-Pacific consensus statement on the management of chronic hepatitis B: a 2012 update. Hepatology International. 2012;6:531–561. doi: 10.1007/s12072-012-9365-4. [DOI] [PubMed] [Google Scholar]
- 43.Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology. 2009;50:661–662. doi: 10.1002/hep.23190. [DOI] [PubMed] [Google Scholar]
- 44.Wu CY, Lin JT, Ho HJ, Su CW, Lee TY, Wang SY, Wu C, Wu JC. Association of nucleos(t)ide analogue therapy with reduced risk of hepatocellular carcinoma in patients with chronic hepatitis B: a nationwide cohort study. Gastroenterology. 2014;147:143–151.e5. doi: 10.1053/j.gastro.2014.03.048. [DOI] [PubMed] [Google Scholar]
- 45.Liaw YF, Raptopoulou-Gigi M, Cheinquer H, Sarin SK, Tanwandee T, Leung N, Peng CY, Myers RP, Brown RS, Jeffers L, et al. Efficacy and safety of entecavir versus adefovir in chronic hepatitis B patients with hepatic decompensation: a randomized, open-label study. Hepatology. 2011;54:91–100. doi: 10.1002/hep.24361. [DOI] [PubMed] [Google Scholar]
- 46.Hosaka T, Suzuki F, Kobayashi M, Seko Y, Kawamura Y, Sezaki H, Akuta N, Suzuki Y, Saitoh S, Arase Y, et al. Long-term entecavir treatment reduces hepatocellular carcinoma incidence in patients with hepatitis B virus infection. Hepatology. 2013;58:98–107. doi: 10.1002/hep.26180. [DOI] [PubMed] [Google Scholar]
- 47.Wong GL, Chan HL, Mak CW, Lee SK, Ip ZM, Lam AT, Iu HW, Leung JM, Lai JW, Lo AO, et al. Entecavir treatment reduces hepatic events and deaths in chronic hepatitis B patients with liver cirrhosis. Hepatology. 2013;58:1537–1547. doi: 10.1002/hep.26301. [DOI] [PubMed] [Google Scholar]
- 48.Su TH, Hu TH, Chen CY, Huang YH, Chuang WL, Lin CC, Wang CC, Su WW, Peng CY, Chien RN, et al. Reduction of hepatocellular carcinoma in hepatitis B-related cirrhosis patients with long-term entecavir therapy - A follow-up report of C-TEAM study. Hepatology. 2014;60:1284a–1285a. [Google Scholar]
- 49.Sievert W, Strasser S, Gane E, George J, Weilert F, Flaherty JF, Dinh P, Schall RA, Martins EB, Yee L, et al. Long term tenofovir disoproxil fumarate (TDF) therapy and the risk of hepatocellular carcinoma. J Gastroen Hepatol. 2013;28:53–54. [Google Scholar]
- 50.Yang HI, Yuen MF, Chan HL, Han KH, Chen PJ, Kim DY, Ahn SH, Chen CJ, Wong VW, Seto WK. Risk estimation for hepatocellular carcinoma in chronic hepatitis B (REACH-B): development and validation of a predictive score. Lancet Oncol. 2011;12:568–574. doi: 10.1016/S1470-2045(11)70077-8. [DOI] [PubMed] [Google Scholar]
- 51.Papatheodoridis GV, Lampertico P, Manolakopoulos S, Lok A. Incidence of hepatocellular carcinoma in chronic hepatitis B patients receiving nucleos(t)ide therapy: a systematic review. J Hepatol. 2010;53:348–356. doi: 10.1016/j.jhep.2010.02.035. [DOI] [PubMed] [Google Scholar]
- 52.Zhang QQ, An X, Liu YH, Li SY, Zhong Q, Wang J, Hu HD, Zhang DZ, Ren H, Hu P. Long-term nucleos(t)ide analogues therapy for adults with chronic hepatitis B reduces the risk of long-term complications: a meta-analysis. Virol J. 2011;8:72. doi: 10.1186/1743-422X-8-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Singal AK, Salameh H, Kuo YF, Fontana RJ. Meta-analysis: the impact of oral anti-viral agents on the incidence of hepatocellular carcinoma in chronic hepatitis B. Aliment Pharmacol Ther. 2013;38:98–106. doi: 10.1111/apt.12344. [DOI] [PubMed] [Google Scholar]
- 54.Sung JJ, Tsoi KK, Wong VW, Li KC, Chan HL. Meta-analysis: Treatment of hepatitis B infection reduces risk of hepatocellular carcinoma. Aliment Pharmacol Ther. 2008;28:1067–1077. doi: 10.1111/j.1365-2036.2008.03816.x. [DOI] [PubMed] [Google Scholar]
- 55.Papatheodoridis GV, Manolakopoulos S, Touloumi G, Vourli G, Raptopoulou-Gigi M, Vafiadis-Zoumbouli I, Vasiliadis T, Mimidis K, Gogos C, Ketikoglou I, et al. Virological suppression does not prevent the development of hepatocellular carcinoma in HBeAg-negative chronic hepatitis B patients with cirrhosis receiving oral antiviral(s) starting with lamivudine monotherapy: results of the nationwide HEPNET. Greece cohort study. Gut. 2011;60:1109–1116. doi: 10.1136/gut.2010.221846. [DOI] [PubMed] [Google Scholar]
- 56.Arends P, Sonneveld MJ, Zoutendijk R, Carey I, Brown A, Fasano M, Mutimer D, Deterding K, Reijnders JG, Oo Y, et al. Entecavir treatment does not eliminate the risk of hepatocellular carcinoma in chronic hepatitis B: limited role for risk scores in Caucasians. Gut. 2014:Epub ahead of print. doi: 10.1136/gutjnl-2014-307023. [DOI] [PubMed] [Google Scholar]
- 57.Yang SC, Lee CM, Hu TH, Wang JH, Lu SN, Hung CH, Changchien CS, Chen CH. Virological response to entecavir reduces the risk of liver disease progression in nucleos(t)ide analogue-experienced HBV-infected patients with prior resistant mutants. J Antimicrob Chemother. 2013;68:2154–2163. doi: 10.1093/jac/dkt147. [DOI] [PubMed] [Google Scholar]
- 58.Zoutendijk R, Reijnders JG, Zoulim F, Brown A, Mutimer DJ, Deterding K, Hofmann WP, Petersen J, Fasano M, Buti M, et al. Virological response to entecavir is associated with a better clinical outcome in chronic hepatitis B patients with cirrhosis. Gut. 2013;62:760–765. doi: 10.1136/gutjnl-2012-302024. [DOI] [PubMed] [Google Scholar]
- 59.Kim SS, Hwang JC, Lim SG, Ahn SJ, Cheong JY, Cho SW. Effect of virological response to entecavir on the development of hepatocellular carcinoma in hepatitis B viral cirrhotic patients: comparison between compensated and decompensated cirrhosis. Am J Gastroenterol. 2014;109:1223–1233. doi: 10.1038/ajg.2014.145. [DOI] [PubMed] [Google Scholar]
- 60.Papatheodoridis GV, Dalekos GN, Yurdaydin C, Buti M, Goulis J, Arends P, Sypsa V, Manolakopoulos S, Mangia G, Gatselis N, et al. Incidence and predictors of hepatocellular carcinoma in Caucasian chronic hepatitis B patients receiving entecavir or tenofovir. J Hepatol. 2015;62:363–370. doi: 10.1016/j.jhep.2014.08.045. [DOI] [PubMed] [Google Scholar]
- 61.Gokturk S, Basar R, Ucar AR, Basgoze BH, Altinkaynak M, Buyukballi P, Alpaslan B, Baran B, Ormeci AC, Soyer OM, et al. Virological Supression Does not Prevent the Development of Hepatocellular Carsinoma in Cirrhotic Patients with Genotype D Hepatitis B Infection. Hepatology. 2013;58:684A–685A. [Google Scholar]
- 62.Yuen MF, Wong DK, Fung J, Ip P, But D, Hung I, Lau K, Yuen JC, Lai CL. HBsAg Seroclearance in chronic hepatitis B in Asian patients: replicative level and risk of hepatocellular carcinoma. Gastroenterology. 2008;135:1192–1199. doi: 10.1053/j.gastro.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 63.Villa E, Fattovich G. No inflammation? No cancer! Clear HBV early and live happily. J Hepatol. 2010;52:768–770. doi: 10.1016/j.jhep.2010.01.018. [DOI] [PubMed] [Google Scholar]
- 64.Simonetti J, Bulkow L, McMahon BJ, Homan C, Snowball M, Negus S, Williams J, Livingston SE. Clearance of hepatitis B surface antigen and risk of hepatocellular carcinoma in a cohort chronically infected with hepatitis B virus. Hepatology. 2010;51:1531–1537. doi: 10.1002/hep.23464. [DOI] [PubMed] [Google Scholar]
- 65.Wong JS, Wong GL, Tsoi KK, Wong VW, Cheung SY, Chong CN, Wong J, Lee KF, Lai PB, Chan HL. Meta-analysis: the efficacy of anti-viral therapy in prevention of recurrence after curative treatment of chronic hepatitis B-related hepatocellular carcinoma. Aliment Pharmacol Ther. 2011;33:1104–1112. doi: 10.1111/j.1365-2036.2011.04634.x. [DOI] [PubMed] [Google Scholar]
- 66.Kim BK, Park JY, Kim do Y, Kim JK, Kim KS, Choi JS, Moon BS, Han KH, Chon CY, Moon YM, et al. Persistent hepatitis B viral replication affects recurrence of hepatocellular carcinoma after curative resection. Liver Int. 2008;28:393–401. doi: 10.1111/j.1478-3231.2007.01625.x. [DOI] [PubMed] [Google Scholar]
- 67.Cheung YS, Chan HL, Wong J, Lee KF, Poon TC, Wong N, Lai PB. Elevated perioperative transaminase level predicts intrahepatic recurrence in hepatitis B-related hepatocellular carcinoma after curative hepatectomy. Asian J Surg. 2008;31:41–49. doi: 10.1016/S1015-9584(08)60056-1. [DOI] [PubMed] [Google Scholar]
- 68.Huang G, Lai EC, Lau WY, Zhou WP, Shen F, Pan ZY, Fu SY, Wu MC. Posthepatectomy HBV reactivation in hepatitis B-related hepatocellular carcinoma influences postoperative survival in patients with preoperative low HBV-DNA levels. Ann Surg. 2013;257:490–505. doi: 10.1097/SLA.0b013e318262b218. [DOI] [PubMed] [Google Scholar]
- 69.Piao CY, Fujioka S, Iwasaki Y, Fujio K, Kaneyoshi T, Araki Y, Hashimoto K, Senoh T, Terada R, Nishida T, et al. Lamivudine treatment in patients with HBV-related hepatocellular carcinoma--using an untreated, matched control cohort. Acta Med Okayama. 2005;59:217–224. doi: 10.18926/AMO/31969. [DOI] [PubMed] [Google Scholar]
- 70.Shuqun C, Mengchao W, Han C, Feng S, Jiahe Y, Wenming C, Zhengfeng Y, Yuxiang Z, Peijun W. Antiviral therapy using lamivudine and thymosin alpha1 for hepatocellular carcinoma coexisting with chronic hepatitis B infection. Hepatogastroenterology. 2006;53:249–252. [PubMed] [Google Scholar]
- 71.Kuzuya T, Katano Y, Kumada T, Toyoda H, Nakano I, Hirooka Y, Itoh A, Ishigami M, Hayashi K, Honda T, et al. Efficacy of antiviral therapy with lamivudine after initial treatment for hepatitis B virus-related hepatocellular carcinoma. J Gastroenterol Hepatol. 2007;22:1929–1935. doi: 10.1111/j.1440-1746.2006.04707.x. [DOI] [PubMed] [Google Scholar]
- 72.Kubo S, Tanaka H, Takemura S, Yamamoto S, Hai S, Ichikawa T, Kodai S, Shinkawa H, Sakaguchi H, Tamori A, et al. Effects of lamivudine on outcome after liver resection for hepatocellular carcinoma in patients with active replication of hepatitis B virus. Hepatol Res. 2007;37:94–100. doi: 10.1111/j.1872-034X.2007.00013.x. [DOI] [PubMed] [Google Scholar]
- 73.Chan AC, Chok KS, Yuen WK, Chan SC, Poon RT, Lo CM, Fan ST. Impact of antiviral therapy on the survival of patients after major hepatectomy for hepatitis B virus-related hepatocellular carcinoma. Arch Surg. 2011;146:675–681. doi: 10.1001/archsurg.2011.125. [DOI] [PubMed] [Google Scholar]
- 74.Li N, Lai EC, Shi J, Guo WX, Xue J, Huang B, Lau WY, Wu MC, Cheng SQ. A comparative study of antiviral therapy after resection of hepatocellular carcinoma in the immune-active phase of hepatitis B virus infection. Ann Surg Oncol. 2010;17:179–185. doi: 10.1245/s10434-009-0694-z. [DOI] [PubMed] [Google Scholar]
- 75.Wu CY, Chen YJ, Ho HJ, Hsu YC, Kuo KN, Wu MS, Lin JT. Association between nucleoside analogues and risk of hepatitis B virus-related hepatocellular carcinoma recurrence following liver resection. JAMA. 2012;308:1906–1914. doi: 10.1001/2012.jama.11975. [DOI] [PubMed] [Google Scholar]
- 76.Yin J, Li N, Han Y, Xue J, Deng Y, Shi J, Guo W, Zhang H, Wang H, Cheng S, et al. Effect of antiviral treatment with nucleotide/nucleoside analogs on postoperative prognosis of hepatitis B virus-related hepatocellular carcinoma: a two-stage longitudinal clinical study. J Clin Oncol. 2013;31:3647–3655. doi: 10.1200/JCO.2012.48.5896. [DOI] [PubMed] [Google Scholar]
- 77.Huang G, Lau WY, Wang ZG, Pan ZY, Yuan SX, Shen F, Zhou WP, Wu MC. Antiviral therapy improves postoperative survival in patients with hepatocellular carcinoma: a randomized controlled trial. Ann Surg. 2015;261:56–66. doi: 10.1097/SLA.0000000000000858. [DOI] [PubMed] [Google Scholar]
- 78.Sun P, Dong X, Cheng X, Hu Q, Zheng Q. Nucleot(s)ide analogues for hepatitis B virus-related hepatocellular carcinoma after curative treatment: a systematic review and meta-analysis. PLoS One. 2014;9:e102761. doi: 10.1371/journal.pone.0102761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhou Y, Zhang Z, Zhao Y, Wu L, Li B. Antiviral therapy decreases recurrence of hepatitis B virus-related hepatocellular carcinoma after curative resection: a meta-analysis. World J Surg. 2014;38:2395–2402. doi: 10.1007/s00268-014-2586-z. [DOI] [PubMed] [Google Scholar]
- 80.Manolakopoulos S, Karatapanis S, Elefsiniotis J, Mathou N, Vlachogiannakos J, Iliadou E, Kougioumtzan A, Economou M, Triantos C, Tzourmakliotis D, et al. Clinical course of lamivudine monotherapy in patients with decompensated cirrhosis due to HBeAg negative chronic HBV infection. Am J Gastroenterol. 2004;99:57–63. doi: 10.1046/j.1572-0241.2003.04021.x. [DOI] [PubMed] [Google Scholar]
- 81.Kumada T, Toyoda H, Tada T, Kiriyama S, Tanikawa M, Hisanaga Y, Kanamori A, Niinomi T, Yasuda S, Andou Y, et al. Effect of nucleos(t)ide analogue therapy on hepatocarcinogenesis in chronic hepatitis B patients: a propensity score analysis. J Hepatol. 2013;58:427–433. doi: 10.1016/j.jhep.2012.10.025. [DOI] [PubMed] [Google Scholar]
- 82.Gordon SC, Lamerato LE, Rupp LB, Li J, Holmberg SD, Moorman AC, Spradling PR, Teshale EH, Vijayadeva V, Boscarino JA, et al. Antiviral therapy for chronic hepatitis B virus infection and development of hepatocellular carcinoma in a US population. Clin Gastroenterol Hepatol. 2014;12:885–893. doi: 10.1016/j.cgh.2013.09.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Coffin CS, Rezaeeaval M, Pang JX, Alcantara L, Klein P, Burak KW, Myers RP. The incidence of hepatocellular carcinoma is reduced in patients with chronic hepatitis B on long-term nucleos(t)ide analogue therapy. Aliment Pharmacol Ther. 2014;40:1262–1269. doi: 10.1111/apt.12990. [DOI] [PubMed] [Google Scholar]
- 84.Yoshida H, Yoshida H, Goto E, Sato T, Ohki T, Masuzaki R, Tateishi R, Goto T, Shiina S, Kawabe T, et al. Safety and efficacy of lamivudine after radiofrequency ablation in patients with hepatitis B virus-related hepatocellular carcinoma. Hepatol Int. 2008;2:89–94. doi: 10.1007/s12072-007-9020-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hung IF, Poon RT, Lai CL, Fung J, Fan ST, Yuen MF. Recurrence of hepatitis B-related hepatocellular carcinoma is associated with high viral load at the time of resection. Am J Gastroenterol. 2008;103:1663–1673. doi: 10.1111/j.1572-0241.2008.01872.x. [DOI] [PubMed] [Google Scholar]
- 86.Koda M, Nagahara T, Matono T, Sugihara T, Mandai M, Ueki M, Ohyama K, Hosho K, Okano J, Kishimoto Y, et al. Nucleotide analogs for patients with HBV-related hepatocellular carcinoma increase the survival rate through improved liver function. Intern Med. 2009;48:11–17. doi: 10.2169/internalmedicine.48.1534. [DOI] [PubMed] [Google Scholar]
- 87.Chuma M, Hige S, Kamiyama T, Meguro T, Nagasaka A, Nakanishi K, Yamamoto Y, Nakanishi M, Kohara T, Sho T, et al. The influence of hepatitis B DNA level and antiviral therapy on recurrence after initial curative treatment in patients with hepatocellular carcinoma. J Gastroenterol. 2009;44:991–999. doi: 10.1007/s00535-009-0093-z. [DOI] [PubMed] [Google Scholar]
- 88.Urata Y, Kubo S, Takemura S, Uenishi T, Kodai S, Shinkawa H, Sakae M, Kaneda K, Ohata K, Nozawa A, et al. Effects of antiviral therapy on long-term outcome after liver resection for hepatitis B virus-related hepatocellular carcinoma. J Hepatobiliary Pancreat Sci. 2012;19:685–696. doi: 10.1007/s00534-011-0489-z. [DOI] [PubMed] [Google Scholar]
- 89.Yang T, Lu JH, Zhai J, Lin C, Yang GS, Zhao RH, Shen F, Wu MC. High viral load is associated with poor overall and recurrence-free survival of hepatitis B virus-related hepatocellular carcinoma after curative resection: a prospective cohort study. Eur J Surg Oncol. 2012;38:683–691. doi: 10.1016/j.ejso.2012.04.010. [DOI] [PubMed] [Google Scholar]
- 90.Huang G, Yang Y, Shen F, Pan ZY, Fu SY, Lau WY, Zhou WP, Wu MC. Early viral suppression predicts good postoperative survivals in patients with hepatocellular carcinoma with a high baseline HBV-DNA load. Ann Surg Oncol. 2013;20:1482–1490. doi: 10.1245/s10434-012-2803-7. [DOI] [PubMed] [Google Scholar]
- 91.Ke Y, Ma L, You XM, Huang SX, Liang YR, Xiang BD, Li LQ, Zhong JH. Antiviral therapy for hepatitis B virus-related hepatocellular carcinoma after radical hepatectomy. Cancer Biol Med. 2013;10:158–164. doi: 10.7497/j.issn.2095-3941.2013.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Su CW, Chiou YW, Tsai YH, Teng RD, Chau GY, Lei HJ, Hung HH, Huo TI, Wu JC. The Influence of Hepatitis B Viral Load and Pre-S Deletion Mutations on Post-Operative Recurrence of Hepatocellular Carcinoma and the Tertiary Preventive Effects by Anti-Viral Therapy. PLoS One. 2013;8:e66457. doi: 10.1371/journal.pone.0066457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yeh YC, Liu CJ, Kuo RN, Lai CL, Shau WY, Chen PJ, Lai MS. Association of adjuvant antiviral therapy with risk of cancer progression and deaths in patients with hepatitis-B-virus-related hepatocellular carcinoma following curative treatment: a nationwide cohort study. PLoS One. 2014;9:e102051. doi: 10.1371/journal.pone.0102051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhang ZY, Zhou ZQ, Zhou GW. Higher efficacy of antiviral therapy after major hepatectomy in patients with hepatitis B virus-related hepatocellular carcinoma of less than 3 cm. Eur J Gastroenterol Hepatol. 2014;26:1116–1124. doi: 10.1097/MEG.0000000000000153. [DOI] [PubMed] [Google Scholar]
- 95.Hann HW, Coben R, Brown D, Needleman L, Rosato E, Min A, Hann RS, Park KB, Dunn S, DiMarino AJ. A long-term study of the effects of antiviral therapy on survival of patients with HBV-associated hepatocellular carcinoma (HCC) following local tumor ablation. Cancer Med. 2014;3:390–396. doi: 10.1002/cam4.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nishikawa H, Nishijima N, Arimoto A, Inuzuka T, Kita R, Kimura T, Osaki Y. Effect of nucleoside analog use in patients with hepatitis B virus-related hepatocellular carcinoma. Hepatol Res. 2014;44:608–620. doi: 10.1111/hepr.12169. [DOI] [PubMed] [Google Scholar]
- 97.Chong CC, Wong GL, Wong VW, Ip PC, Cheung YS, Wong J, Lee KF, Lai PB, Chan HL. Antiviral therapy improves post-hepatectomy survival in patients with hepatitis B virus-related hepatocellular carcinoma: a prospective-retrospective study. Aliment Pharmacol Ther. 2015;41:199–208. doi: 10.1111/apt.13034. [DOI] [PubMed] [Google Scholar]
- 98.Miao RY, Zhao HT, Yang HY, Mao YL, Lu X, Zhao Y, Liu CN, Zhong SX, Sang XT, Huang JF. Postoperative adjuvant antiviral therapy for hepatitis B/C virus-related hepatocellular carcinoma: a meta-analysis. World J Gastroenterol. 2010;16:2931–2942. doi: 10.3748/wjg.v16.i23.2931. [DOI] [PMC free article] [PubMed] [Google Scholar]


