Abstract
Previous studies demonstrated that interleukin-1β (IL-1β) and nerve growth factor (NGF) increase synthesis of substance P (SP) in airway neurons both after ozone (O3) exposure and by direct application. It was postulated that NGF mediates O3-induced IL-1β effects on SP. The current study specifically focused on the influence of O3 on IL-1β, NGF, and SP levels in mice bronchoalveolar lavage fluid (BALF) and whether these mediators may be linked in an inflammatory-neuronal cascade in vivo. The findings showed that in vivo O3 exposure induced an increase of all three proteins in mouse BALF and that O3-induced elevations in both NGF and SP are mediated by the inflammatory cytokine IL-1β. Further, inhibition of NGF reduced O3 induced increases of SP in both the lung BALF and lung tissue, demonstrating NGF serves as a mediator of IL-1β effects on SP. These data indicate that IL-1β is an early mediator of O3-induced rise in NGF and subsequent SP release in mice in vivo.
Ozone (O3) is one of six criterion air pollutants included in the Clean Air Act and regulated by the U.S. Environmental Protection Agency (EPA) National Ambient Air Quality Standard (U.S. EPA, 2013). Ground-level O3 is generated through reactions between nitrogen oxides and volatile organic compounds released into the atmosphere predominantly from vehicle exhausts and industrial and power-generating facilities. When inhaled, O3 interacts with airway epithelial cells, increasing epithelial permeability and stimulating release of cytokines and other inflammatory mediators (Bhalla, 1999; McCullough et al., 2014) that stimulate sensory nerves (Taylor-Clark and Undem, 2010). Among inflammatory mediators released from epithelial cells by ozone inhalation are interleukin-1β (IL-1β; (Wu et al., 2012; Johnston et al., 2007)) and nerve growth factor (NGF; Graham et al., 2001; Hunter et al., 2011). Excitation of sensory nerve terminals in the airways not only generates action potentials triggering airway reflexes (Joad et al., 1996b) but also releases neuropeptides, including substance P (SP), which is chemotactic for inflammatory cells and increases permeability of bronchial vessels (Baluk et al., 1998).
SP release from airway sensory nerve terminals mediates airway hyperresponsiveness and inflammation, referred to as neurogenic inflammation (Barnes, 1986; Baluk et al., 1992; Borson et al., 1989). Once SP is released, it is rapidly degraded by neutral endopeptidase and is not recycled back into the nerve terminal (Nadel, 1991; Umeno et al., 1989). Therefore, sustained actions of SP release require increased synthesis by translation of preprotachykinin (PPT) mRNA (Krause et al., 1987). Allergens and inhaled irritants increase the PPT gene in airway C-fiber neurons (Hunter et al., 2000a; Fischer et al., 1996). The airway epithelium synthesizes and releases NGF in close proximity to the intraepithelial sensory nerve fibers also located within the epithelial layer (Hunter et al., 2011).
While both IL-1 and NGF signaling are associated with SP upregulation and synthesis in the airways, these mediators have not been shown to operate in a coordinated sequence in an in vivo system of air pollution exposure such as O3. In this study, it was postulated that enhanced SP release from sensory neurons during O3 exposure is attributed to O3 induced IL-1β released in airways, stimulating NGF release, which then increases SP levels in sensory neurons. The rationale for the experiments is that inhibition of NGF might reduce SP response and inhibition of IL-1β may attenuate both NGF and SP release in the airways after O3 exposure.
METHODS
Animal Use and Anesthetics
Adult (8 wk old, 50–60 g) male ICR mice (Harlan Laboratories, Inc.) were housed 4 per cage, under controlled light cycle (12-h light/dark) and temperature (22–24°C) conditions, with access to food and water ad libitum in the West Virginia University animal facility. Mice were anesthetized with a ketamine/xylazine mixture (25 mg/kg and 2 mg/kg, respectively) in a single intraperitoneal (ip) injection before all intratracheal (i.t.) instillations of IL-1β, IL-1β receptor antagonist (IL-1Ra) or anti-NGF. Animals were euthanized 24 h after O3/air exposure or i.t. instillations with a lethal dose of sodium pentobarbital (200 mg/kg). All procedures were approved through ACUC review under Protocol 06-0501.
Experimental Design
Four different experimental protocols were used in the study.
Effect of ozone exposure on IL-1β, NGF, and SP release in BALF. This study established baseline values for IL-1β, NGF and SP after ozone exposure. Six groups of mice were exposed to 2 ppm ozone or filtered air (FA). The number of mice was different for each group: for ozone groups, n = 4, 6, and 4 for IL-1β, NGF, and SP respectively; for FA groups, n = 6, 5, and 3, respectively. Bronchoalveaolar lavage fluid (BALF) was obtained 24 h postexposure. Levels of IL-1β, NGF, and SP were determined by enzyme-linked immunosorbent assay (ELISA).
Instillation of IL-1β. This study aimed to determine the influence of IL-1β on NGF and SP levels in BALF. Four groups of anesthetized mice received 20 μl of 2 μg/ml i.t. instillation IL-1β (catalogue number 15271 Sigma-Aldrich, St. Louis, MO) or vehicle (saline) for a dose of 600 μg/kg as previously reported by Wu et al (2002). BALF was collected 24 h postexposure and NGF and SP levels were measured by ELISA. Mice in these experiments were not exposed to O3. The number of mice for NGF/saline measurements was five and for SP/saline was six.
Effect of IL-1β receptor antagonist (IL-1Ra) on ozone-induced changes in NGF and SP in BALF. This study determined the effect of IL-1β inhibition on the O3-induced changes in NGF and SP measured in BALF. Four groups of anesthetized mice received i.t. instillation consisting of 20 ul of 200 ng/ml IL- Ra (gift from Amgen, Inc., Thousand Oaks, CA) for a dose of 4 ng/mouse or 20 μl saline 30 min prior to O3 exposure based on our previous study (Wu et al., 2008). Twenty-four hours after O3 exposure, NGF and SP in BALF were measured by ELISA based on previous studies. NGF was measured in six mice in both the IL- Ra and saline groups, five in the SP group receiving IL-Ra, and four in the SP group receiving saline.
Effect of NGF antibody on ozone-induced levels of SP in BALF and lung homogenate. These studies were conducted to examine the influence of neutralizing NGF on SP levels in BALF and in lung homogenates. Two groups of anesthetized mice were treated with 0.2 ml of 1:2000 rabbit anti-mouse NGF antibody or rabbit immunoglobulin (Ig) G (both 3 μg protein/ml; catalogue number N6655, Sigma-Aldrich, St. Louis, MO) by a subcutaneous (sc) injection (Cardenas et al., 2010). Then 30 min later, the same mice were exposed to 1 ml aerosolized solution containing 3 μg/ml anti-NGF or IgG diluted 1:2000. The combined sc and aerosol strategies to inhibit NGF were found to be effective in assessing NPY expression after cigarette smoke exposure in fetal mouse (Wu et al., 2012). The aerosol exposures were conducted in a Plexiglas chamber (15 × 15 × 10 cm), which was connected to a mini ultrasonic nebulizer with an output rate of 0.1 ml/min for 10 min. One hour after the end of the aerosol, mice were exposed to 2 ppm O3 for 3 h. Controls received both sc injection and aerosol exposure to nonimmune rabbit IgG in the same doses and were exposed to O3 1 h after aerosol treatment. There were five mice in each group.
In Vivo Ozone Exposure
All in vivo O3 exposures were conducted at 2 ppm in a 12 × 12 inch stainless-steel and glass chamber for 3 h at room temperature. The exposure to 2 ppm for 3 h was selected because exposure parameters in this range produce robust neural and inflammatory responses after 24 h in animal models (Shore et al., 2002; Wu et al., 2008; Vancza et al., 2009). Ozone was produced by passing hospital-grade air through a drying and high-efficiency particle (HEPA) filter and then through an ultraviolet light source. The O3 concentration in the chamber was measured by chemiluminescence with a calibrated O3 analyzer (OA 350-2R model, Forney Corporation; Carrolton, TX) sampled by a probe located 6 inches from the breathing zone of the mice opposite the O3 delivery port. Air control animals were exposed to filtered air using procedures identical to those just described, except O3 was not delivered to the mixing chamber. The temperature and humidity in the exposure chamber remained at 28°C and 50%, respectively. The O3 exposure apparatus was described in detail in a previous paper (Wu et al., 2002).
Bronchoalveolar Lavage Fluid (BALF) Collection
Lungs of euthanized mice were lavaged with 3 ml phosphate-buffered saline (PBS; 1.5 ml, twice) through a tracheal cannula and BALF was placed into tubes with 30 μl of proteinase inhibitor phosphoramidon (1 × 10−4 μM) to inhibit neutral endopeptidases that degrade SP. The collected BAL fluid was centrifuged at 1200 × g for 10–12 min at 15°C. The supernatant was aliquoted and frozen at −80°C for subsequent assays.
Lung Tissue Homogenates
Lung tissue homogenates were used in the NGF inhibition experiment to measure SP that might be stored but not released from nerve terminals. It was considered that NGF inhibition may affect not only SP synthesis, but also release due to potential attenuation of TRPV1 receptors that are known to be regulated by NGF (Zhang et al., 2005). Therefore, in a separate group of mice, lungs were removed 24 h after O3 exposure, weighed, homogenized, and centrifuged (40,000 × g). The supernatant fractions were collected, filtered, and frozen at −80°C for subsequent SP assays.
Enzyme-Linked Immunosorbent Assay (ELISA)
IL-1β ELISA
BALF supernatant samples (initial 3ml) were frozen at −80°C. The concentration of IL-1β in each sample was assayed using the mouse IL-1β/IL-1F2 DuoSet® ELISA Development System (R&D Systems, Inc.; Minneapolis, MN; sensitivity 31–1000 pg/ml) according to the manufacturer’s instructions.
NGF ELISA
BALF supernatant samples (initial 3 ml) were frozen at −80°C. The concentration of NGF in each sample was assayed using the NGF Emax ImmunoAssay System (sensitivity 7.8–1000 pg/ml, Promega, Madison, WI) according to the manufacturer’s instructions.
SP ELISA
BALF supernatant samples (initial 3 ml) were frozen at −80°C. The concentration of SP in each sample was assayed using the Parameter Substance P Assay (sensitivity 39–2500 pg/ml; R&D Systems, Inc., Minneapolis, MN) according to the manufacturer’s instructions.
Data Analysis
Unless otherwise stated, results are expressed as means ± SE. Statistical analysis was performed using one-way analysis of variance (ANOVA) with multiple comparisons. When the main effect was considered significant at p < .05, pairwise comparisons were made with a post hoc analysis (Fisher’s least significant difference). A value of p = .05 was considered significant and n represents the number of animals studied.
RESULTS
Effect of Ozone on IL-1β, NGF, and SP Levels in BALF
Experiments were conducted to evaluate the effects of O3 on IL-1β, NGF, and SP protein levels in BALF in comparison to filtered-air (FA) counterparts. BALF was collected 24 h after O3 or FA exposure. Ozone exposure (Figures 1A, 1B, and 1C) significantly increased levels of IL-1β (210.3 ± 38 pg/ml, n = 4), NGF (3230.7 ± 1039 pg/ml, n = 6), and SP (4280.3 ± 635 pg/ml, n = 4) in BALF when compared to FA exposure groups (114.6 ± 9.8 pg/ml, n = 6; 753.3 ± 217 pg/ml, n = 5; and 1488.7 ± 459 pg/ml, n = 3, respectively).
FIGURE 1.
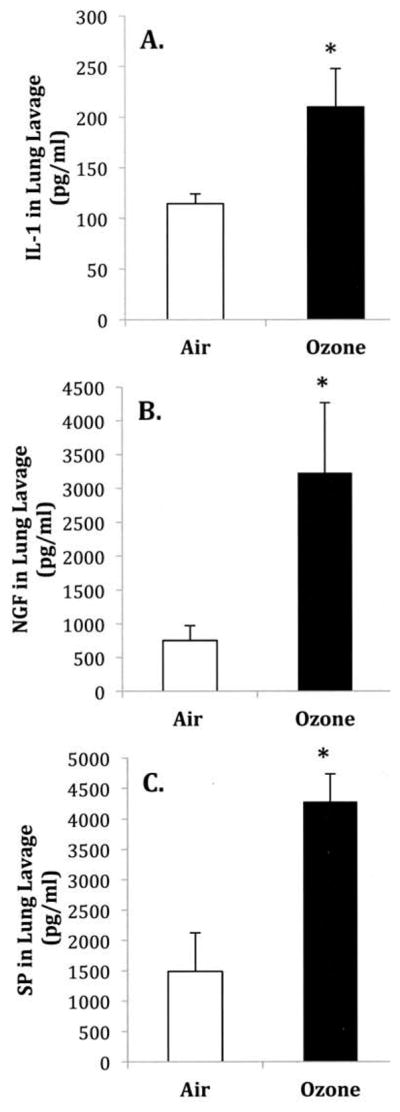
The effect of O3 exposure on (A) IL-1β, (B) NGF, and (C) SP protein levels in bronchoalveolar lavage fluid. Protein levels were measured by ELISA 24 h after filtered air (FA) or O3 (2 ppm, 3 h) exposure. (A) NGF levels, (B) NGF levels, and (C) SP levels. Values are means ± SE of 4–6 mice/group. Asterisk indicates significant difference between FA- and O3-exposed animals, p ≤ .05.
Effect of Exogenous IL-1β on NGF and SP Levels in BALF
Experiments were conducted to determine whether i.t. instillation of exogenous IL-1β might alter NGF and SP levels in BALF collected 24 h after instillation. Neither group was exposed to O3. NGF levels were significantly increased from 1349.7 ± 463 pg/ml in saline to 3424.8 ± 915 pg/ml in IL-1β-treated animals (n = 5 for both groups, Figure 2A). BALF from IL-1β-treated animals contained a significantly higher concentration of SP, increasing from 1557.8 ± 440 pg/ml in control to 4970.7 ± 61 pg/ml in IL-1β-treated animals (n = 6 for both groups, Figure 2B).
FIGURE 2.
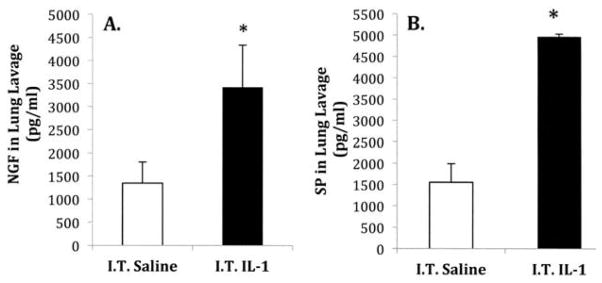
Influence of intra-tracheal instillation of exogenous IL-1β treatment on (A) NGF and (B) SP protein levels in bronchoalveolar lavage fluid. Protein levels were measured by ELISA 24 h after saline or IL-1β (200 μl/ml) treatment. (A) NGF levels and (B) SP levels in lung lavage fluid. Values are means ± SE of 5–6 mice/group. Asterisk indicates significant difference between NGF levels in saline- and IL-1β-treated animals, p ≤ .05.
Effect of IL-1 Ra on Ozone-Induced Changes in NGF and SP Levels in BALF
Experiments were undertaken to examine whether i.t. instillation of IL-1 receptor antagonist (Ra) might block O3-induced changes in levels of NGF and SP in BALF. Mice were administered i.t. instillation of IL-1 Ra or saline (vehicle) prior to air or O3 exposures. The levels of NGF and SP in BALF were measured 24 h following air or O3 treatment. Mice receiving IL-Ra prior to O3 exposure demonstrated significantly lower levels of NGF and SP in BALF compared to animals receiving saline (vehicle) (Figures 3A and 3B). The NGF and SP levels in saline treated mice prior to O3 exposure were 3696.5 ± 681 pg/ml (n = 6) and 4959 ± 1038 pg/ml (n = 4), respectively. After instillation of IL-Ra, NGF levels in O3 exposed animals were decreased to 2070 ± 371 pg/ml (n = 6) and SP levels to 1852.2 ± 567 pg/ml (n = 5).
FIGURE 3.
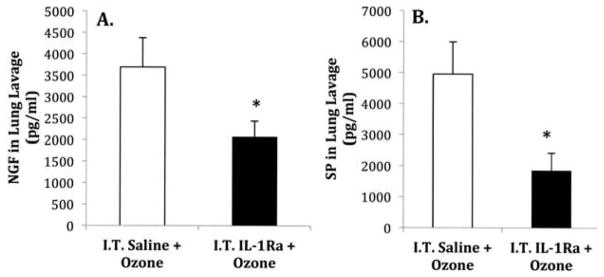
Effect of IL-1 receptor antagonist on O3 induced changes in NGF and SP levels in bronchoalveolar lavage fluid. Animals were instilled with saline or IL-Ra (200 ng/ml) prior to O3 exposure. NGF and SP levels were measured in bronchoalveolar lavage fluid by an ELISA 24 h after O3 exposure (2 ppm, 3 h). (A) NGF levels and (B) SP levels in the lung lavage. Values are means ± SE of 4–6 mice/group. Asterisk indicates significant difference in NGF and SP levels between saline-and IL-1 Ra-treated animals, p ≤ .05.
Effect of NGF-Ab on Ozone-Induced Changes in SP Levels in BALF and Lung Tissue
Further experiments were conducted to determine whether an NGF antibody (NGF-Ab) might block O3-induced SP changes in BALF and lung tissue. Animals (n = 5 per group) were exposed to both aerosolized and sc injection of NGF-Ab or IgG prior to O3 exposure and SP levels in BALF and lung homogenates were measured 24 h after O3. Pretreatment with NGF-Ab prior to O3 exposure significantly lowered levels of SP in BALF (Figure 4A) and lung tissue (Figure 4B) compared to animals treated with IgG. The SP levels in BALF fell from 6654 ± 73.68 pg/ml in the IgG-O3 treatment group to 5880 ± 98.43 pg/ml in the NGF-Ab-O3 treatment group. The SP levels in the lung tissue decreased from 2082 ± 187 pg/ml with IgG-O3 to 1295 ± 201 pg/ml with NGF-Ab-O3.
FIGURE 4.
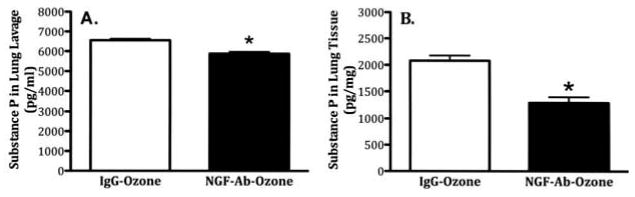
Influence of NGF-Ab on O3-induced changes in SP levels in bronchoalveolar lavage fluid and lung tissue. Animals were instilled with IgG or NGF-Ab (both at 1:2000 dilution, 4 ml/kg) prior to O3 exposure. SP levels were measured in bronchoalveolar lavage fluid and lung tissue by an ELISA 24 h after O3 exposure (2 ppm, 3 h). (A) SP levels in lung lavage; (B) SP levels in the lung tissue. Values are means ± SE of 5 mice/group. Asterisk indicates significant difference in SP levels between IgG- and NGF-Ab-treated animals in both lung lavage and lung tissue (p ≤ .05).
DISCUSSION
Data demonstrated that in vivo O3exposure significantly increased the concentrations of IL-1β, NGF, and SP in mouse BALF and SP in lung tissue. All three of these mediators are associated with airway inflammation in other animal models of O3 exposure and epidemiological studies (Lee et al., 1979; Woolf et al., 1994; Koto et al., 1995; Bonham et al., 1996; Joad et al., 1996a; Wu et al., 1997; Braun et al., 1998; Barnes, 2001; Graham et al., 2001; Bachar et al., 2004; Nassenstein et al., 2004; Park et al., 2004; Johnson et al., 2005; de Vries et al., 2006; Krasteva et al., 2011). The unique contribution of the current study demonstrates that SP production after O3 exposure is dependent on IL-1β-stimulated release of NGF.
The finding that IL-1β is a key modulator of SP release in the airways is consistent with our previous reports of in vivo and in vitro studies in ferrets (Wu et al., 2001, 2003), where O3-induced IL-1β release after O3 exposure enhanced airway responsiveness by modulating SP levels. The present study further confirms the role of IL-1β as a key intermediate signaling molecule in the downstream effects of O3 exposure by demonstrating its modulatory effects on NGF. Previously, Hunter et al. (2011) demonstrated that i.t. instillation of NGF is sufficient to induce increased SP expression in sensory neurons projecting to the airways The present study shows that immunological reduction of O3-induced NGF levels attenuates SP release even in the presence of elevated IL-1β (i.e., ozone exposure). Previous studies reported that IL-1β modulates NGF expression in vitro (Virchow et al., 1998; Olgart and Frossard, 2001; Pons et al., 2001; Freund et al., 2002). NGF and IL-1β levels are increased in asthma (Bonini et al., 1996; Kassel et al., 2001; Olgart-Hoglund et al., 2002). The present findings indicate that IL-1β is directly responsible for the subsequent rise in NGF in airway lavage and that enhanced O3-induced SP expression depends on IL-1β-stimulated release of NGF and subsequent stimulation of SP release by NGF. These observations support a role for NGF, and not IL-1β, as the direct mediator of O3-induced SP elevation in airways.
Previous investigations showed that IL-1β (Wu et al., 2008), NGF (Graham et al., 2001; Hunter et al., 2011), and SP (Fischer et al., 1996; Hunter et al., 2000a; Carr et al., 2002; Sikora et al., 2003; Wilfong and Dey, 2004) are all elevated in the airways during exposures to allergens and environmental irritants. Further, the inflammatory cytokine interleukin 1β (IL-1β) induces the expression of NGF (Fox et al., 2001; Pons et al., 2001) and SP (Wu et al., 2008; Skoff et al., 2009) in neurons. Intratracheal application of NGF increases synthesis of SP in C fiber airway neurons that normally contain SP and induces SP production in A-delta airway neurons that are normally devoid of SP (Hunter et al., 2000b, 2011). These findings suggest that airway irritants induce a signaling sequence involving initial release of IL-1β from the epithelium, IL-1β-stimulated epithelial production of NGF, and NGF-trkA receptor binding on airway sensory nerve terminals leading to upregulated SP synthesis within the neuron. A proposed signaling pathway for NGF-stimulated SP upregulation involves binding of NGF to the trkA receptor located in sensory nerve terminals, internalization of the NGF-trkA complex, formation and retrograde transport of the signaling endosome to the nucleus, and activation of specific signaling pathways affecting translation and SP synthesis (Yu et al., 2011).
NGF is classified as a neurotrophin, but there is increasing evidence suggesting it is involved in a variety of immune functions. NGF was even implicated as an asthma mediator, and in this study direct evidence of inhibiting or blocking NGF reduced SP levels that were induced by O3 exposure, suggesting that NGF contributes to the O3-induced rise in SP. In addition, the source and regulation of NGF expression in airways are not fully understood, but a direct action of NGF on neurons may be part of this response since NGF elevates the number of immunoreactive nerve fibers and neuropeptide content in the airway (Adler et al., 1984; Lindsay and Harmar, 1989; Vedder et al., 1993; Cho et al., 1996; Hoyle et al., 1998; Hunter et al., 2000b; Malcangio et al., 2000; Skoff et al., 2003).
Ozone is a ubiquitous air pollutant shown to produce numerous adverse respiratory effects (Lippmann, 1989; Beckett, 1991; Bhalla, 1999). Of these effects, airway inflammation and airway hyperreactivity (AHR) are the hallmark pulmonary characteristics of O3-mediated exposure. This was observed in animal models and humans. For example, human subjects who show sensitivity to O3 exposure display bronchial hyperresponsiveness to methacholine challenge not observed in nonsensitive subjects (Schelegle et al., 2007). Asthmatics demonstrated increased inflammation markers in nasal lavage fluid after low-level O3 challenge (0.12–0.24 ppm) compared to nonasthmatic control subjects (McBride et al., 1994). A recent study reported that airway allergic hyperreactivity in a mouse model is attributable to activation of airway sensory neurons in vagal ganglia and occurs independent of immune mechanisms (Trankner et al., 2014). The current study specifically investigated a possible signaling pathway responsible for translating O3 exposure to neurogenic inflammation in airways through IL-1β-stimulated NGF release and subsequent NGF-induced upregulation of the sensory neuropeptide SP, a mediator proven to mediate neurogenic inflammation in the airways (Lundberg et al., 1983). In conclusion, this study demonstrated that the signaling pathway for enhanced SP release after O3 exposure involves IL-1β-induced upregulation of NGF and then NGF-induced increase in SP release.
Acknowledgments
FUNDING
This study was supported by the National Institutes of Health (NIH), RO1 HL 80566.
References
- Adler JE, Kessler JA, Black IB. Development and regulation of substance P in sensory neurons in vitro. Dev Biol. 1984;102:417–425. doi: 10.1016/0012-1606(84)90206-9. [DOI] [PubMed] [Google Scholar]
- Bachar O, Adner R, Uddman R, Cardell LO. Nerve growth factor enhances cholinergic innervation and contractile responses to electric field stimulation in a murine in vitro model of chronic asthma. Clin Exp Allergy. 2004;34:1137–1145. doi: 10.1111/j.1365-2222.2004.1868.x. [DOI] [PubMed] [Google Scholar]
- Baluk P, Bolton P, Hirata A, Thurston G, McDonald DM. Endothelial gaps and adherent leukocytes in allergen-induced early- and late-phase plasma leakage in rat airways. Am J Pathol. 1998;152:1463–1476. [PMC free article] [PubMed] [Google Scholar]
- Baluk P, Nadel JA, McDonald DM. Substance P-immunoreactive sensory axons in the rat respiratory tract: A quantitative study of their distribution and role in neurogenic inflammation. J Comp Neurol. 1992;319:586–598. doi: 10.1002/cne.903190408. [DOI] [PubMed] [Google Scholar]
- Barnes PJ. Neural control of human airways in health and disease. Am Rev Respir Dis. 1986;134:1289–1314. doi: 10.1164/arrd.1986.134.5.1289. [DOI] [PubMed] [Google Scholar]
- Barnes PJ. Neurogenic inflammation in the airways. Respir Physiol. 2001;125:145–154. doi: 10.1016/s0034-5687(00)00210-3. [DOI] [PubMed] [Google Scholar]
- Beckett WS. Ozone, air pollution, and respiratory health. Yale J Biol Med. 1991;64:167–175. [PMC free article] [PubMed] [Google Scholar]
- Bhalla DK. Ozone-induced lung inflammation and mucosal barrier disruption: Toxicology, mechanisms, and implications. J Toxicol Environ Health B. 1999;2:31–86. doi: 10.1080/109374099281232. [DOI] [PubMed] [Google Scholar]
- Bonham AC, Kott KS, Joad JP. Sidestream smoke exposure enhances rapidly adapting receptor responses to substance P in young guinea pigs. J Appl Physiol. 1996;81:1715–1722. doi: 10.1152/jappl.1996.81.4.1715. [DOI] [PubMed] [Google Scholar]
- Bonini S, Lambiase A, Angelucci F, Magrini L, Manni L, Aloe L. Circulating nerve growth factor levels are increased in humans with allergic diseases and asthma. Proc Natl Acad Sci USA. 1996;93:10955–10960. doi: 10.1073/pnas.93.20.10955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borson DB, Brokaw JJ, Sekizawa K, McDonald DM, Nadel JA. Neutral endopeptidase and neurogenic inflammation in rats with respiratory infections. J Appl Physiol. 1989;66:2653–2658. doi: 10.1152/jappl.1989.66.6.2653. [DOI] [PubMed] [Google Scholar]
- Braun A, Appel E, Baruch R, Herz U, Botchkarev V, Paus R, Brodie C, Renz H. Role of nerve growth factor in a mouse model of allergic airway inflammation and asthma. Eur J Immunol. 1998;28:3240–3251. doi: 10.1002/(SICI)1521-4141(199810)28:10<3240::AID-IMMU3240>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Cardenas S, Scuri M, Samsell L, Ducatman B, Bejarano P, Auais A, Doud M, Mathee K, Piedimonte G. Neurotrophic and neuroimmune responses to early-life Pseudomonas aeruginosa infection in rat lungs. Am J Physiol Lung Cell Mol Physiol. 2010;299:L334–L344. doi: 10.1152/ajplung.00017.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr MJ, Hunter DD, Jacoby DB, Undem BJ. Expression of tachykinins in nonnociceptive vagal afferent neurons during respiratory viral infection in guinea pigs. Am J Respir Crit Care Med. 2002;165:1071–1075. doi: 10.1164/ajrccm.165.8.2108065. [DOI] [PubMed] [Google Scholar]
- Cho HJ, Park EH, Bae MA, Kim JK. Expression of mRNAs for preprotachykinin and nerve growth factor receptors inthe dorsal root ganglion following peripheral inflammation. Brain Res. 1996;716:197–201. doi: 10.1016/0006-8993(96)00026-1. [DOI] [PubMed] [Google Scholar]
- de Vries A, Engels F, Henricks PA, Leusink-Muis T, McGregor GP, Braun A, Groneberg DA, Dessing MC, Nijkamp FP, Fischer A. Airway hyper-responsiveness in allergic asthma in guineapigs is mediated by nerve growth factor via the induction of substance P: A potential role for trkA. Clin Exp Allergy. 2006;36:1192–1200. doi: 10.1111/j.1365-2222.2006.02549.x. [DOI] [PubMed] [Google Scholar]
- Fischer A, McGregor GP, Saria A, Philippin B, Kummer W. Induction of tachykinin gene and peptide expression in guinea pig nodose primary afferent neurons by allergic airway inflammation. J Clin Invest. 1996;98:2284–2291. doi: 10.1172/JCI119039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox AJ, Patel HJ, Barnes PJ, Belvisi MG. Release of nerve growth factory by human pulmonary epithelial cells: Role in airway inflammatory diseases. Eur J Pharmacol. 2001;424:159–162. doi: 10.1016/s0014-2999(01)01138-4. [DOI] [PubMed] [Google Scholar]
- Freund V, Pons F, Joly V, Mathieu E, Martinet N, Frossard N. Upregulation of nerve growth factor expression by human airway smooth muscle cells in inflammatory conditions. Eur Respir J. 2002;20:458–463. doi: 10.1183/09031936.02.00269202. [DOI] [PubMed] [Google Scholar]
- Graham RM, Friedman M, Hoyle GW. Sensory nerves promote ozone-induced lung inflammation in mice. Am J Respir Crit Care Med. 2001;164:307–313. doi: 10.1164/ajrccm.164.2.2007115. [DOI] [PubMed] [Google Scholar]
- Hoyle GW, Graham RM, Finkelstein JB, Nguyen KP, Gozal D, Friedman M. Hyperinnervation of the airways in transgenic mice overexpressing nerve growth factor. Am J Respir Cell Mol Biol. 1998;18:149–157. doi: 10.1165/ajrcmb.18.2.2803m. [DOI] [PubMed] [Google Scholar]
- Hunter DD, Carrell-Jacks LA, Batchelor TP, Dey RD. Role of nerve growth factor in ozone-induced neural responses in early postnatal airway development. Am J Respir Cell Mol Biol. 2011;45:359–365. doi: 10.1165/rcmb.2010-0345OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter DD, Fedan JS, Satterfield BE, Huang J, Dey RD. Toluene diisocyanate enhances substance P in sensory neurons innervating the nasal mucosa. Am J Respir Crit Care Med. 2000a;161:543–549. doi: 10.1164/ajrccm.161.2.9812083. [DOI] [PubMed] [Google Scholar]
- Hunter DD, Myers AC, Undem BJ. Nerve growth factor-induced phenotypic switch in guinea pig airway sensory neurons. Am J Respir Crit Care Med. 2000b;161:1985–1990. doi: 10.1164/ajrccm.161.6.9908051. [DOI] [PubMed] [Google Scholar]
- Joad JP, Avadhanam KP, Watt KC, Kott KS, Bric JM, Pinkerton KE. Effects of extended sidestream smoke exposure on components of the C-fiber axon reflex. Toxicology. 1996a;112:195–203. doi: 10.1016/0300-483x(96)03403-8. [DOI] [PubMed] [Google Scholar]
- Joad JP, Kott KS, Bric JM. The local C-fiber contribution to ozone-induced effects on the isolated guinea pig lung. Toxicol Appl Pharmacol. 1996b;141:561–567. doi: 10.1006/taap.1996.0323. [DOI] [PubMed] [Google Scholar]
- Johnson VJ, Yucesoy B, Luster MI. Prevention of IL-1 signaling attenuates airway hyperresponsiveness and inflammation in a murine model of toluene diisocyanate-induced asthma. J Allergy Clin Immunol. 2005;116:851–858. doi: 10.1016/j.jaci.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Johnston RA, Mizgerd JP, Flynt L, Quinton LJ, Williams ES, Shore SA. Type I interleukin-1 receptor is required for pulmonary responses to subacute ozone exposure in mice. Am J Respir Cell Mol Biol. 2007;37:477–484. doi: 10.1165/rcmb.2006-0315OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassel O, de Blay F, Duvernelle C, Olgart C, Israel-Biet D, Krieger P, Moreau L, Muller C, Pauli G, Frossard N. Local increase in the number of mast cells and expression of nerve growth factor in the bronchus of asthmatic patients after repeated inhalation of allergen at low-dose. Clin Exp Allergy. 2001;31:1432–1440. doi: 10.1046/j.1365-2222.2001.01177.x. [DOI] [PubMed] [Google Scholar]
- Koto H, Aizawa H, Takata S, Inoue H, Hara N. An important role of tachykinins in ozone-induced airway hyper-responsiveness. Am J Respir Crit Care Med. 1995;151:1763–1769. doi: 10.1164/ajrccm.151.6.7767518. [DOI] [PubMed] [Google Scholar]
- Krasteva G, Canning BJ, Hartmann P, Veres TZ, Papadakis T, Muhlfeld C, Schliecker K, Tallini YN, Braun A, Hackstein H, Baal N, Weihe E, Schutz B, Kotlikoff M, Ibanez-Tallon I, Kummer W. Cholinergic chemosensory cells in the trachea regulate breathing. Proc Natl Acad Sci USA. 2011;108:9478–9483. doi: 10.1073/pnas.1019418108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause JE, Chirgwin JM, Carter MS, Xu ZS, Hershey AD. Three rat preprotachykinin mRNAs encode the neuropeptides substance P and neurokinin A. Proc Natl Acad Sci USA. 1987;84:881–885. doi: 10.1073/pnas.84.3.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee LY, Dumont C, Djokic TD, Menzel TE, Nadel JA. Mechanism of rapid, shallow breathing after ozone exposure in the conscious dogs. J Appl Physiol. 1979;46:1108–1109. doi: 10.1152/jappl.1979.46.6.1108. [DOI] [PubMed] [Google Scholar]
- Lindsay RM, Harmar AJ. Nerve growth factor regulates expression of neuropeptide genes in adult sensory neurons. Nature. 1989;337:362–364. doi: 10.1038/337362a0. [DOI] [PubMed] [Google Scholar]
- Lippmann M. Health effects of ozone. A critical review. J Air Pollut Control Assoc. 1989;39:672–695. doi: 10.1080/08940630.1989.10466554. [DOI] [PubMed] [Google Scholar]
- Lundberg JM, Saria A, Brodin E, Rosell S, Folkers K. A substance P antagonist inhibits vagally induced increase in vascular permeability and bronchial smooth muscle contraction in the guinea pig. Proc Natl Acad Sci USA. 1983;80:1120–1124. doi: 10.1073/pnas.80.4.1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malcangio M, Ramer MS, Jones MG, McMahon SB. Abnormal substance P release from the spinal cord following injury to primary sensory neurons. Eur J Neurosci. 2000;12:397–399. doi: 10.1046/j.1460-9568.2000.00946.x. [DOI] [PubMed] [Google Scholar]
- McBride DE, Koenig JQ, Luchtel DL, Williams PV, Henderson WR., Jr Inflammatory effects of ozone in the upper airways of subjects with asthma. Am J Respir Crit Care Med. 1994;149:1192–1197. doi: 10.1164/ajrccm.149.5.8173759. [DOI] [PubMed] [Google Scholar]
- McCullough SD, Duncan KE, Swanton SM, Dailey LA, Diaz-Sanchez D, Devlin RB. Ozone induces a pro-inflammatory response in primary human bronchial epithelial cells through MAP kinase activation without NF-kappaB activation. Am J Respir Cell Mol Biol. 2014;51:426–435. doi: 10.1165/rcmb.2013-0515OC. [DOI] [PubMed] [Google Scholar]
- Nadel JA. Role of enzymes from inflammatory cells on airway submucosal gland secretion. Respiration. 1991;58(suppl 1):3–5. doi: 10.1159/000195961. [DOI] [PubMed] [Google Scholar]
- Nassenstein C, Kerzel S, Braun A. Neurotrophins and neurotrophin receptors in allergic asthma. Prog Brain Res. 2004;146:347–367. doi: 10.1016/S0079-6123(03)46022-6. [DOI] [PubMed] [Google Scholar]
- Olgart C, Frossard N. Human lung fibroblasts secrete nerve growth factor: Effect of inflammatory cytokines and glucocorticoids. Eur Respir J. 2001;18:115–121. doi: 10.1183/09031936.01.00069901. [DOI] [PubMed] [Google Scholar]
- Olgart-Hoglund C, de Blay F, Oster JP, Duvernelle C, Kassel O, Pauli G, Frossard N. Nerve growth factor levels and localisation in human asthmatic bronchi. Eur Respir J. 2002;20:1110–1116. doi: 10.1183/09031936.02.00205402. [DOI] [PubMed] [Google Scholar]
- Park JW, Taube C, Swasey C, Kodama T, Joetham A, Balhorn A, Takeda K, Miyahara N, Allen CB, Dakhama A, Kim SH, Dinarello CA, Gelfand EW. Interleukin-1 receptor antagonist attenuates airway hyperresponsiveness following exposure to ozone. Am J Respir Cell Mol Biol. 2004;30:830–836. doi: 10.1165/rcmb.2003-0373OC. [DOI] [PubMed] [Google Scholar]
- Pons F, Freund V, Kuissu H, Mathieu E, Olgart C, Frossard N. Nerve growth factor secretion by human lung epithelial A549 cells in pro- and anti-inflammatory conditions. Eur J Pharmacol. 2001;428:365–369. doi: 10.1016/s0014-2999(01)01280-8. [DOI] [PubMed] [Google Scholar]
- Schelegle ES, Walby WF, Adams WC. Time course of ozone-induced changes in breathing pattern in healthy exercising humans. J Appl Physiol. 2007;102:688–697. doi: 10.1152/japplphysiol.00141.2006. [DOI] [PubMed] [Google Scholar]
- Shore SA, Johnston RA, Schwartzman IN, Chism D, Krishna Murthy GG. Ozone-induced airway hyperresponsiveness is reduced in immature mice. J Appl Physiol. 2002;92:1019–1028. doi: 10.1152/japplphysiol.00381.2001. [DOI] [PubMed] [Google Scholar]
- Sikora ER, Stone S, Tomblyn S, Castranova V, Dey RD. Asphalt exposure enhances neuropeptides levels in sensory neurons projecting to the nasal epithelium. J Toxicol Environ Health A. 2003;66:1015–1027. doi: 10.1080/15287390306394. [DOI] [PubMed] [Google Scholar]
- Skoff AM, Resta C, Swamydas M, Adler JE. Nerve growth factor (NGF) and glial cell line-derived neurotrophic factor (GDNF) regulate substance P release in adult spinal sensory neurons. Neurochem Res. 2003;28:847–854. doi: 10.1023/a:1023211107073. [DOI] [PubMed] [Google Scholar]
- Skoff AM, Zhao C, Adler JE. Interleukin-1alpha regulates substance P expression and release in adult sensory neurons. Exp Neurol. 2009;217:395–400. doi: 10.1016/j.expneurol.2009.03.022. [DOI] [PubMed] [Google Scholar]
- Taylor-Clark TE, Undem BJ. Ozone activates airway nerves via the selective stimulation of TRPA1 ion channels. J Physiol. 2010;588:423–433. doi: 10.1113/jphysiol.2009.183301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trankner D, Hahne N, Sugino K, Hoon MA, Zuker C. Population of sensory neurons essential for asthmatic hyperreactivity of inflamed airways. Proc Natl Acad Sci USA. 2014;111:11515–11520. doi: 10.1073/pnas.1411032111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umeno E, Nadel JA, Huang HT, McDonald DM. Inhibition of neutral endopeptidase potentiates neurogenic inflammation in the rat trachea. J Appl Physiol. 1989;66:2647–2652. doi: 10.1152/jappl.1989.66.6.2647. [DOI] [PubMed] [Google Scholar]
- U.S. Environmental Protection Agency. Integrated science assessment of ozone and related photochemical oxidants (Final report) 2013 EPA/600/R-10/076F. http://www.epa.gov/ncea/isa/index.htm.
- Vancza EM, Galdanes K, Gunnison A, Hatch G, Gordon T. Age, strain, and gender as factors for increased sensitivity of the mouse lung to inhaled ozone. Toxicol Sci. 2009;107:535–543. doi: 10.1093/toxsci/kfn253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vedder H, Affolter HU, Otten U. Nerve growth factor (NGF) regulates tachykinin gene expression and biosynthesis in rat sensory neurons during early postnatal development. Neuropeptides. 1993;24:351–357. doi: 10.1016/0143-4179(93)90006-v. [DOI] [PubMed] [Google Scholar]
- Virchow JC, Julius P, Lommatzsch M, Luttmann W, Renz H, Braun A. Neurotrophins are increased in bronchoalveolar lavage fluid after segmental allergen provocation. Am J Respir Crit Care Med. 1998;158:2002–2005. doi: 10.1164/ajrccm.158.6.9803023. [DOI] [PubMed] [Google Scholar]
- Wilfong ER, Dey RD. Nerve growth factor and substance P regulation in nasal sensory neurons after toluene diisocyanate exposure. Am J Respir Cell Mol Biol. 2004;30:793–800. doi: 10.1165/rcmb.2003-0303OC. [DOI] [PubMed] [Google Scholar]
- Woolf CJ, Safieh-Garabedian B, Ma QP, Crilly P, Winter J. Nerve growth factor contributes to the generation of inflammatory sensory hypersensitivity. Neruoscience. 1994;62:327–331. doi: 10.1016/0306-4522(94)90366-2. [DOI] [PubMed] [Google Scholar]
- Wu ZX, Barker JS, Batchelor TP, Dey RD. Interleukin (IL)-1 regulates ozone-enhanced tracheal smooth muscle responsiveness by increasing substance P (SP) production in intrinsic airway neurons of ferret. Respir Physiol Neurobiol. 2008;164:300–311. doi: 10.1016/j.resp.2008.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu ZX, Benders KB, Hunter DD, Dey RD. Early postnatal exposure of mice to side-steam tobacco smoke increases neuropeptide Y in lung. Am J Physiol Lung Cell Mol Physiol. 2012;302:L152–L159. doi: 10.1152/ajplung.00071.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu ZX, Maize DF, Jr, Satterfield BE, Frazer DG, Fedan JS, Dey RD. Role of intrinsic airway neurons in ozone-induced airway hyperresponsiveness in ferret trachea. J Appl Physiol. 2001;91:371–378. doi: 10.1152/jappl.2001.91.1.371. [DOI] [PubMed] [Google Scholar]
- Wu ZX, Morton RF, Lee LY. Role of tachykinins in ozone-induced airway hyperresponsiveness to cigarette smoke in guinea pigs. J Appl Physiol. 1997;83:958–965. doi: 10.1152/jappl.1997.83.3.958. [DOI] [PubMed] [Google Scholar]
- Wu ZX, Satterfield BE, Dey RD. Substance P released from intrinsic airway neurons contributes to ozone-enhanced airway hyperresponsiveness in ferret trachea. J Appl Physiol. 2003;95:742–750. doi: 10.1152/japplphysiol.00109.2003. [DOI] [PubMed] [Google Scholar]
- Wu ZX, Satterfield BE, Fedan JS, Dey RD. Interleukin-1beta-induced airway hyperresponsiveness enhances substance P in intrinsic neurons of ferret airway. Am J Physiol Lung Cell Mol Physiol. 2002;283:L909–L917. doi: 10.1152/ajplung.00363.2001. [DOI] [PubMed] [Google Scholar]
- Yu T, Calvo L, Anta B, Lopez-Benito S, Southon E, Chao MV, Tessarollo L, Arevalo JC. Regulation of trafficking of activated TrkA is critical for NGF-mediated functions. Traffic. 2011;12:521–534. doi: 10.1111/j.1600-0854.2010.01156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Huang J, McNaughton PA. NGF rapidly increases membrane expression of TRPV1 heat-gated ion channels. EMBO J. 2005;24:4211–4223. doi: 10.1038/sj.emboj.7600893. [DOI] [PMC free article] [PubMed] [Google Scholar]


