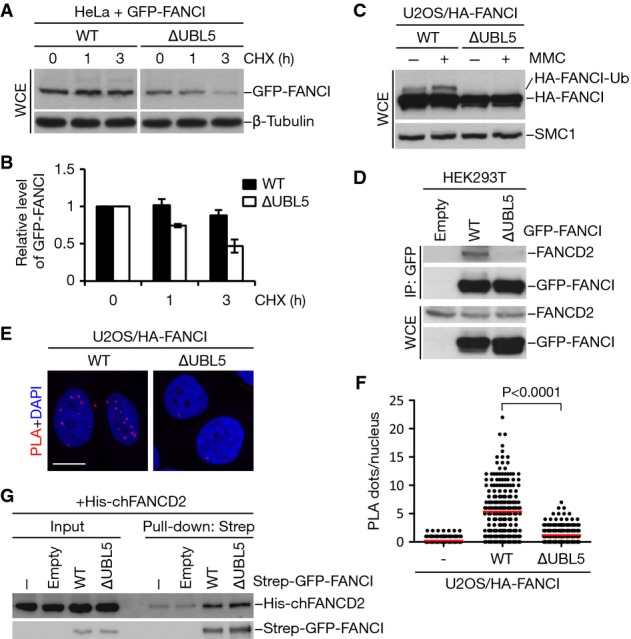
HeLa cells transfected with GFP-tagged FANCI WT or ∆UBL5 were harvested at the indicated times following addition of cycloheximide (CHX). Cell lysates were immunoblotted with GFP and β-tubulin antibodies.
Quantification of GFP-FANCI levels in (A) by image analysis, normalized to β-tubulin levels. Mean values (± SD) from three independent experiments are shown.
U2OS cells stably expressing HA-tagged FANCI WT or ∆UBL5 were treated or not with 1 μM mitomycin C (MMC) for 16 h. Cell extracts were analyzed by immunoblotting with HA and SMC1 antibodies.
Extracts of HEK293T cells transfected with the indicated GFP-FANCI constructs were subjected to GFP immunoprecipitation followed by immunoblotting with FANCD2 and GFP antibodies.
U2OS cells stably expressing HA-FANCI WT or ∆UBL5 were fixed and incubated with antibodies against HA and FANCD2. The interaction between HA-FANCI and FANCD2 was visualized using PLA. Nuclei were stained with DAPI. Representative images are shown. Scale bar, 10 μm.
Quantification of data in (E). Graph shows the quantification of PLA dots per nucleus. More than 200 cells were counted for each condition. Red lines indicate the mean of the data plotted. P-value was calculated by the Mann–Whitney U-test.
Strep-GFP-FANCI WT or ∆UBL5 was purified from HEK293T cells and incubated with bacterially produced, recombinant His6-FANCD2. Complexes were immobilized on Strep-Tactin Sepharose and washed extensively. Bound material and input samples were then subjected to immunoblotting with His6 and GFP antibodies.