Figure 1.
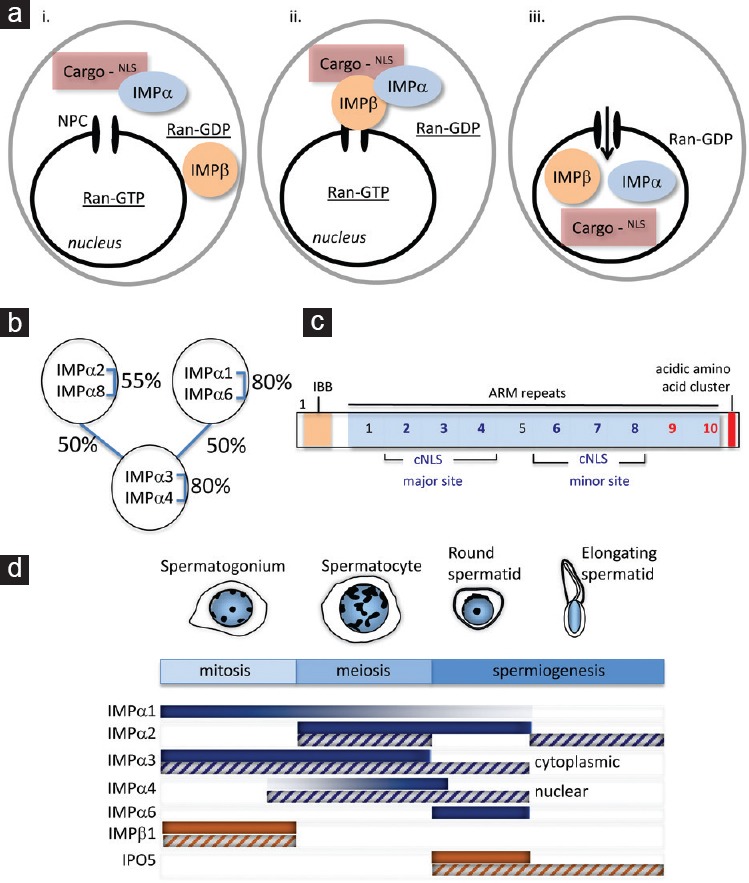
Synthesis of importin proteins that function in nucleocytoplasmic transport is developmentally regulated during spermatogenesis. (a) Classical nucleocytoplasmic transport. Proteins larger than 40 kDa that undertake key functions in the nucleus require active transport through the nuclear pore complex (NPC). For many proteins, such movement is mediated by members of the importin family. (i) Importin α (IMPα) proteins act as receptors that recognize nuclear localization signal (NLS) motifs in the cargo proteins. (ii) Complex formation with importin β1 (IMPβ) allows the passage of cargo molecules through the nuclear pore. Other IMPβ family members, including IPO5, can mediate IMPα-independent transport. (iii) Once in the nucleus, the complex is dissociated, releasing the cargo protein to play its nuclear role, and the importin proteins are recycled back into the cytoplasm. Maintenance of the RanGDP/GTP gradient, in which RanGTP is high in the nucleus, is critical for regulated nucleocytoplasmic transport. (b) Three clades of importin α proteins. The importin α (IMPα) proteins are classified into three distinct subgroups based on percentage identity, as indicated. Despite this classification, there is significant (50%) identity across subgroups. (c) IMPα proteins harbor multiple domains that mediate cargo binding. The classical localization signal (cNLS) classes recognized by the six murine IMPa cargo proteins correspond to either mono- or bipartite sequences of 8–10 basic residue-enriched amino acids. These sequences occur in the major and/or minor binding grooves within the ARM repeat motifs and can be identified with reasonable accuracy by the cNLS Mapper algorithm.42 However, cargo binding to a C-terminal “acidic” domain has been identified.13 This domain was recently examined through a new algorithm designed specifically to identify common amino acid sequence patterns within the set of binding proteins that mediate binding to the C-terminus of IMPα proteins. This newly defined motif has been designated iCBS, for ‘IMPα C-terminal binding segment’.32 IBB, importin β binding domain. ARM, armadillo. (d) Importins are highly regulated at both the RNA and protein level, and each has a specific pattern of expression that correlates with spermatogenic progression. The major developmental stages of spermatogenesis are shown, with the mitotic, meiotic, and spermiogenic phases indicated. Based on studies in rodent models, importin transcript (solid bars) and protein (hatched bars) levels are presented by shading intensity, indicating a potential role as drivers of germ cell development through adult spermatogenesis. IMPα3 and IMPα4 are noted, as despite their high degree of similarity (80%), they are each detected in different stages of spermatogenesis, and in different subcellular compartments.
