Figure 3.
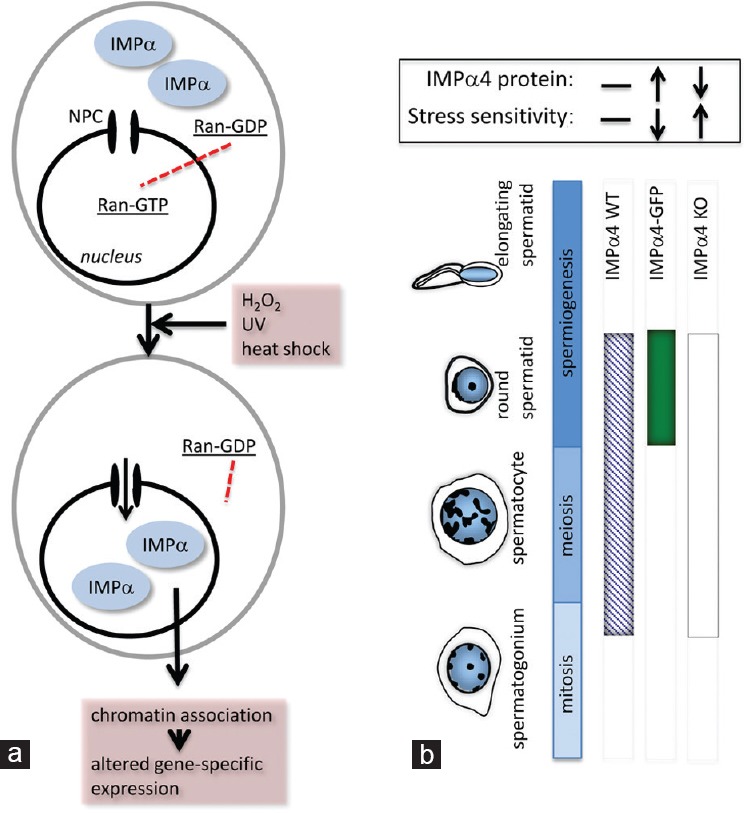
Importin function in cellular stress. (a) There is a well-defined association between changes in importin protein localization and function and the responses of cells to stress. In cultured cells, exposure to stresses that include oxidative stress (H2O2), ultraviolet light (UV) and heat shock, leads to rapid IMPα nuclear accumulation. This altered IMPα subcellular localization is considered to result from the collapsing RanGTP–GDP gradient, which results from the stress-induced decrease in cellular ATP levels. In the absence of high nuclear RanGTP, IMPα-mediated nuclear export and import halt. Significantly, these nuclear-located IMPa proteins associate strongly with chromatin, and are thought to directly induce specific alterations in gene expression.25,26 (b) Examination of mouse models with altered importin expression revealed a role for IMPα4 protein in haploid germ cell stress responses. Three mouse strains were compared to assess this: wild type (IMPα4 WT; presence of IMPα4 protein indicated as a blue hatched band), transgenic mice overexpressing IMPα4 with enhanced green fluorescent protein (EGFP) under the protamine promoter for exclusive expression in post-meiotic germ cells (IMPα4-EGFP; solid green band), and an IMPα4 complete knockout line (IMPα4-KO; white band). When testicular germ cells were exposed to high levels of oxidative stress through H2O2, the haploid germ cells–which express IMPα4-EGFP– exhibited increased resistance to stress-induced cell death. Conversely, the absence of IMPα4 led to increased sensitivity in this population. This indicates that IMPα4 aids in protecting the male germline against stress.27
