Abstract
Actin polymerization and development of hyperactivated (HA) motility are two processes that take place during sperm capacitation. Actin polymerization occurs during capacitation and prior to the acrosome reaction, fast F-actin breakdown takes place. The increase in F-actin during capacitation depends upon inactivation of the actin severing protein, gelsolin, by its binding to phosphatydilinositol-4, 5-bisphosphate (PIP2) and its phosphorylation on tyrosine-438 by Src. Activation of gelsolin following its release from PIP2 is known to cause F-actin breakdown and inhibition of sperm motility, which can be restored by adding PIP2 to the cells. Reduction of PIP2 synthesis inhibits actin polymerization and motility, while increasing PIP2 synthesis enhances these activities. Furthermore, sperm demonstrating low motility contained low levels of PIP2 and F-actin. During capacitation there was an increase in PIP2 and F-actin levels in the sperm head and a decrease in the tail. In spermatozoa with high motility, gelsolin was mainly localized to the sperm head before capacitation, whereas in low motility sperm, most of the gelsolin was localized to the tail before capacitation and translocated to the head during capacitation. We also showed that phosphorylation of gelsolin on tyrosine-438 depends upon its binding to PIP2. Stimulation of phospholipase C, by Ca2+-ionophore or by activating the epidermal-growth-factor-receptor, inhibits tyrosine phosphorylation of gelsolin and enhances enzyme activity. In conclusion, these data indicate that the increase of PIP2 and/or F-actin in the head during capacitation enhances gelsolin translocation to the head. As a result, the decrease of gelsolin in the tail allows the maintenance of high levels of F-actin in this structure, which is essential for the development of HA motility.
To acquire the ability to fertilize the egg, mammalian spermatozoa must undergo several biochemical and morphological changes in the female reproductive tract, collectively called capacitation. These changes include cAMP/PKA activation, cholesterol efflux from the plasma membrane, PKA-dependent protein tyrosine phosphorylation, actin polymerization and the development of HA motility.1,2 In a recent study we suggested a mechanism by which the Ser/Thr targeting PKA can lead to Tyr phosphorylation of proteins in the capacitation process. We showed that PKA activates Src which, in turn, inhibits the phosphatase PP1, leading to CaMKII activation, which activates Pyk2 to phosphorylate phosphatidyl-inositol-3-kinase on tyr-458.3
We have shown elsewhere that actin polymerization must take place in order to capacitate spermatozoa while very fast depolymerization of F-actin occurs prior to the acrosome reaction.4 It has been suggested that an increase in F-actin during capacitation creates a network in the sperm head between the plasma membrane and the outer acrosomal membrane, and the dispersion of this F-actin must occur to enable acrosomal exocytosis.4,5,6,7 The increase in F-actin in the sperm tail during capacitation is important for the development of HA motility.8 The latter is characterized by an increase in flagellar bending amplitude and an increase in average lateral head movement.9,10 It was shown that the efficiency of HA sperm to penetrate the egg is much higher than non-HA sperm.11 Sperm motility and HA motility are mediated by PLD-dependent actin polymerization.8 It is known that phosphatidylinositol 4,5-bisphosphate (PIP2) is a cofactor for PLD activation in many cell types.12,13,14,15,16 PIP2 comprises only 1% of plasma membrane phospholipids, however its extraordinary versatility puts it in the center of plasma membrane dynamics governing cell motility, adhesion, endo- and exocytosis.17,18
PIP2 serves as an effector of several proteins such as Myristoylated alanine-rich C-kinase substrate (MARCKS), gelsolin, PLD and PI3K. These proteins are present in spermatozoa19,20 and are involved in the regulation of sperm capacitation and/or the acrosome reaction. PIP2 binds gelsolin and release it from actin filaments ends, exposing sites for actin assembly.21 We have shown that the release of bound gelsolin from PIP2, causes rapid Ca2+-dependent F-actin depolymerization as well as an increased acrosome reaction.22 We have also shown that PIP2 and gelsolin are involved in regulating sperm motility and the development of HA motility.23
LOCALIZATION OF ACTIN AND GELSOLIN BEFORE AND AFTER CAPACITATION
It is shown in Figure 1 and Table 1 that the cellular level of F-actin in human spermatozoa is relatively low before capacitation but is significantly enhanced during capacitation mainly in the sperm head but also in the tail. Prior to the acrosome reaction (AR) fast dispersion of F-actin occurs and this process allows the outer acrosomal membrane and the overlying plasma membrane to interact and fuse to culminate in the AR. Gelsolin, an actin -severing protein, is localized to the G-actin (actin monomers) fraction before capacitation but to the F-actin (filamentous actin) fraction at the end of the capacitation.22 Moreover, there is a significant increase in Ca2+-dependent gelsolin translocation to the sperm head during capacitation concomitantly with the increase in F-actin.
Figure 1.
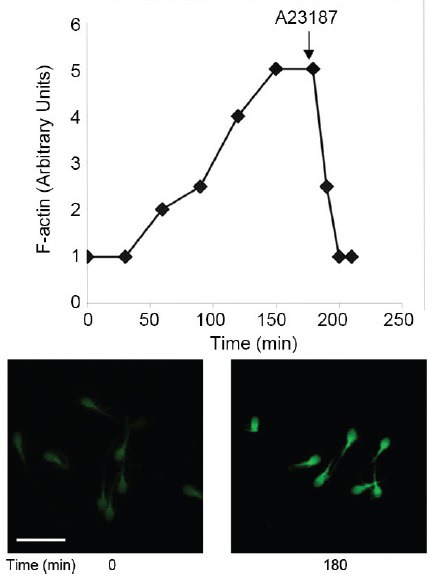
Actin remodeling in sperm capacitation and prior to acrosome reaction: human spermatozoa were incubated under capacitation conditions in Ham's F-10 medium and after 3 h of incubation the Ca2+-ionophore A23187 was added (upper figure). At the indicated times cell samples were stained for F-actin using FITC-phalloidin, photographed under fluorescence microscope, and analysed for fluorescence intensity in the sperm cells (upper figure). A picture of the stained cells is seen in the lower figure.
Table 1.
Effect of various factors on actin modulation during sperm capacitation
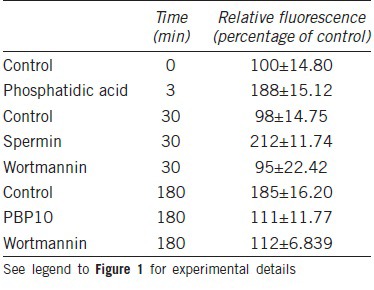
F-actin is also increased in the sperm tail during capacitation, and is thought to be required for the development of HA motility.8 Thus it is likely that there are two reasons for the translocation of gelsolin from the tail to the head; first gelsolin is required in the head to depolymerize F-actin to allow the occurrence of the AR and second the exclusion of gelsolin from the tail prevents F-actin depolymerization in the tail allowing the development of HA motility. It is known that PIP2 (4,5) binds gelsolin and we suggest that this binding keeps gelsolin in an inactive/phosphorylated form allowing F-actin formation during capacitation.22 We found an increase in PIP2 in the sperm head during capacitation simultaneously to the increase in F-actin and gelsolin in the head. These findings support our hypothesis that PIP2 elevation in the sperm head keeps gelsolin inactive and enables the assembly of F-actin in the head. Interestingly, examination of gelsolin location in ejaculated human sperm with high motility revealed high level of gelsolin in the head, while in human spermatozoa with poor motility, a large proportion of gelsolin was localized in the tail and translocated to the sperm head only after capacitation.23 Moreover, releasing of gelsolin from binding to PIP2 by activation of phosphlipase C (PLC) to hydrolyse PIP2 or using PBP10, a peptide derived from PIP2-binding domain of gelsolin which competes with gelsolin binding to PIP2, induced the reverse translocation of gelsolin from the head to the tail. Also, inhibition of PIP2 synthesis prevented the translocation of gelsolin to the head while enhancing PIP2 synthesis significantly increased it.23 Furthermore, we found that tyr-438 phosphorylation/inhibition of gelsolin is reduced when PIP2 levels are decreased and vice versa.24 These data indicate that the cellular levels of PIP2 regulate gelsolin phosphorylation and activation.
ACTIVATION OF GELSOLIN IN CAPACITATED SPERM
Gelsolin activation is regulated by calcium ions, phosphoinositides18,24,25 and by Src-dependent phosphorylation on tyr-438.26 Low concentration of calcium ions cause conformational changes in the C-terminus of gelsolin, which expose its F-actin binding site while higher calcium levels cause a second conformational change exposing the catalytic site.27 In human spermatozoa, activation of gelsolin by enhancing intracellular calcium concentration or by using the peptide PBP10 causes fast depolymerization of F-actin and induction of the AR in capacitated sperm.22 In Sertoli cells, the hydrolysis of PIP2 by PLC resulted in the release the bound gelsolin and its activation.28 A scheme describing the physiological activation of PLC is seen in Figure 2. It is well known that activation of EGFR by EGF or elevation of intra-spermatozoal [Ca2+] using Ca2+-ionophore activates PLCγ.22 In Table 2 we show that EGF or Ca2+-ionophore induces fast F-actin depolymerization and the AR while both are blocked by inhibiting PLC. The induction of F-actin breakdown or AR by PBP10 is not affected by inhibiting PLC, indicating the specificity of PLC inhibition to PIP2 hydrolysis.
Figure 2.
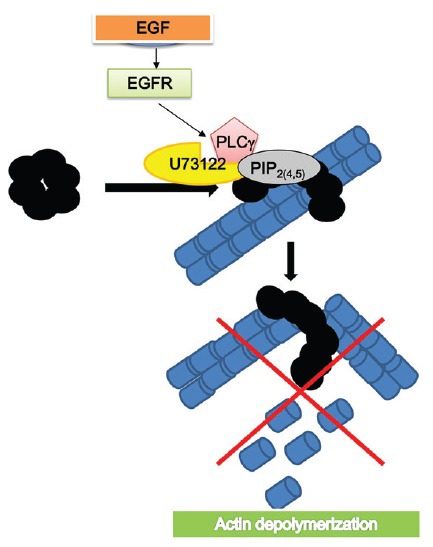
PIP2 and phopholiase C mediate actin modulation: binding of gelsolin to PIP2 causes its inactivation. Activation of PLCγ by the epidermal-grwth-factor-receptor (EGFR) leads to PIP2 hydrolysis and the release of bound gelsolin, which activates and causes F-actin depolymerization.
Table 2.
Effect of various factors of F-actin dispersion and the AR
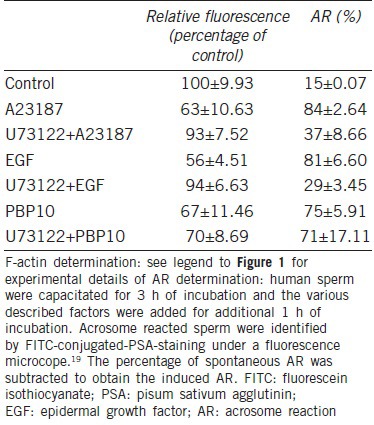
It is well known that there is high increase in intracellular Ca2+ concentrations as a result of the interaction between capacitated sperm and the egg zona pellucida.29 This increase in calcium is essential for the occurrence of the AR which is known to be mediated by PLC activity.30 Interestingly, activation of gelsolin by PBP10 in capacitated sperm, which induces the AR, occurs under conditions in which intracellular Ca2+ concentration is relatively low, further indicating that the increase in intracellular Ca2+ is essential for the activation of PLC to hydrolyse PIP2 leading to the release of bound gelsolin, thereby activating this molecule and stimulating the depolymerization of F-actin, an essential step for the AR to occur.
GELSOLIN ACTIVITY IS INHIBITED DURING CAPACITATION
Since F-actin levels are increased during capacitation, it is likely that gelsolin is inactive during this period of time. Gelsolin can be inhibited by its Src-dependent phosphorylation on tyr-438 and/or it's binding to PIP2, two processes that occur during sperm capacitation. In human sperm, Src is involved in regulating capacitation, Ca fluxes, protein tyrosine phosphorylation and the AR.31,32,33 Previous studies suggested that Src is not directly involved in protein tyrosine phosphorylation but rather inhibits protein phosphatase resulting in an increase in tyrosine phosphorylation of proteins.34 It was shown elsewhere that capacitation is regulated by two parallel pathways, activation of PKA activates Src leading to inactivation of Ser/Thr phosphatase.32,35 (Figure 3). We have recently shown that activation of Src by PKA, inhibits the Ser/Thr phosphatase PP1 resulting in CaMKII activation leading to activation of Pyk2 which phosphorylates PI3K on tyrosine-845.3 Src was found in sperm tail and head and is localized to the membrane fraction,35 similar to gelsolin localization and consistent with our assumption about its inactivating function. These assumptions were verified when Src-dependent phosphorylation of gelsolin on tyr-438 during sperm capacitation was found.23 In addition, we found that activation of PKA/Src caused F-actin formation, which was inhibited by inhibiting Src activity,22 suggesting that activation of Src causes gelsolin inhibition and an increase in F-actin. Also, PBP10-induced F-actin depolymerization is inhibited by activating Src or by inhibition of tyrosine-phosphatase suggesting that although gelsolin is released from its binding to PIP2, it is still highly phosphorylated and inactive. The levels of tyr-p-gelsolin are enhanced by elevating the cellular levels of PIP2 and vice versa,23 suggesting that binding of gelsolin to PIP2 increase its phosphorylation/inhibition. These findings are further supported by showing a decrease in tyr-p-gelsolin by activation of PLC to hydrolyse PIP2 or by releasing gelsolin from PIP2 using PBP10. These observations also suggest that free tyr-p-gelsolin is more sensitive to tyr-phosphatase activity compared to the PIP2-bound p-gelsolin. Also, activation of sperm EGFR caused PLC-dependent dephosphorylation of p-gelsolin, after 1 h of incubation under capacitation conditions.24 These results confirm our hypothesis that gelsolin is inhibited during capacitation due to its binding to PIP2 and tyr-phosphorylation by Src.
Figure 3.
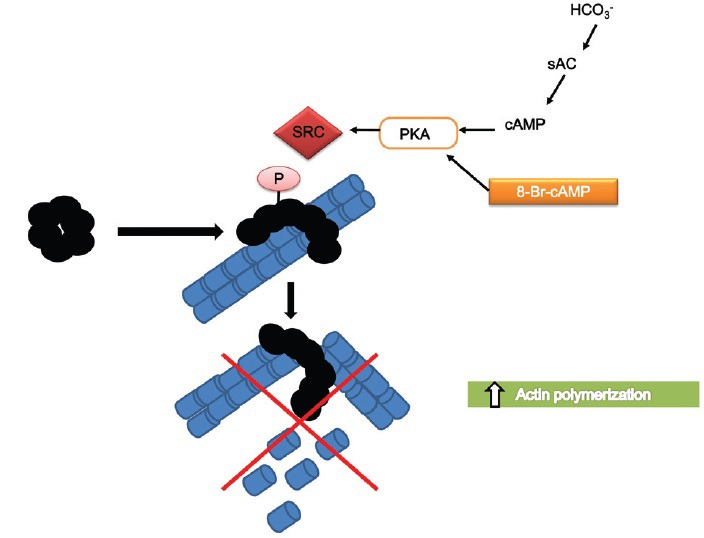
Inactivation of gelsolin by Src-dependent tyrosine phosphorylation: the tyrosine kinase Src is activated by the HCO3−/sAC/cAMP/PKA system leading to gelsolin phosphorylation on Tyr-483 which is stimulated by gelsolin binding to PIP2. Under these conditions p-gelsolin is inactive and actin polymerization occurs.
In conclusion, our data suggest the following model (Figures 4 and 5): the relatively small increase in [Ca2+]i during capacitation leads to conformational changes in gelsolin revealing the F-actin binding site. This change and the increase in F-actin and PIP2 in the sperm head, result in gelsolin being translocated to this region of the cell. Nevertheless, the elevation of PIP2 levels and PKA/Src activation, maintain gelsolin in a phosphorylated/inactivated state and actin polymerization occurs. The increase in F-actin in the tail leads to the development of hyper-activated motility as part of the capacitation process. Immediately prior to the AR (Figure 5), the egg zona-pellucida activates GPCR36 and/or the EGFR37 leading to an increase in [Ca2+]i, and activation of PLC to hydrolyse PIP2, resulting in the release of PIP2-bound p-gelsolin which undergoes dephosphorylation/activation by tyrosine-phosphatase, leading to F-actin dispersion in the head and the occurrence of the AR.
Figure 4.
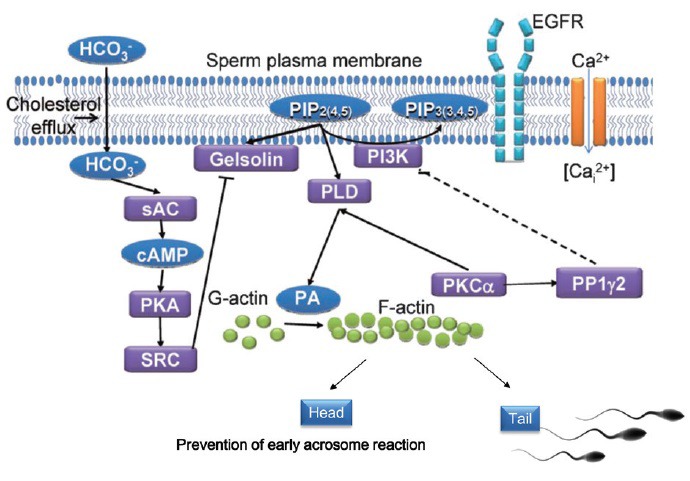
A model describing the biochemical cascade in sperm capacitation: intracellular HCO3− activates sAC to generate cAMP leading to PKA activation and cholesterol efflux from the sperm plasma membrane which further stimulate the HCO3−/sAC/cAMP/PKA cascade. PKA activates Src to phosphorylate/inactivate PIP2-bound gelsolin. PIP2 is a cofactor for PLD activation and this activation is stimulated by PKCα, leading to phosphatidylcholine hydrolysis and production of phosphatidic acid (PA) which mediates the conversion of G-actin to F-actin. Thus, activation of PLD and prevention of F-actin dispersion by inhibiting gelsolin, allows F-actin formation. F-actin in the head prevents immature acrosome reaction and in the tail F-actin regulates sperm motility including HA motility.
Figure 5.
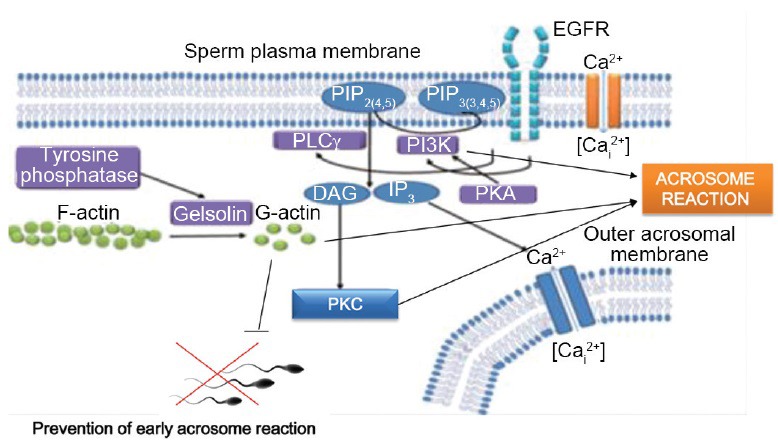
A model describing the biochemical cascade that leads to the acrosome reaction (AR): the binding of capacitated spermatozoa to the egg zona pellucida causes a fast and high increase in intra-spermatozoal Ca2+ concentration. As a result, active PLC catalyses PIP2 hydrolysis to produce IP3 and diacylglycerol (DAG) and the release of bound p-gelsolin. The p-gelsolin undergoes dephosphorylation/activation by tyrosine phosphatase leadin to conversion of F-actin to G-actin. IP3 activates Ca2+ channel in the outer acrosomal membrane which reduces intra-acrosomal Ca2+ leading to the activation of Ca2+- dependent-Ca2+ channel in the plasma membrane which causes further increase in intracellular Ca2+ which together with DAG activate PKC which mediates the acrosome reaction. Also, PKA-dependent PI3K activation occurs towards the end of the capacitation process, involves in the mechanism leding to the AR.
REFERENCES
- 1.Visconti PE. Understanding the molecular basis of sperm capacitation through kinase design. Proc Natl Acad Sci U S A. 2009;106:667–8. doi: 10.1073/pnas.0811895106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buffone MG, Wertheimer EV, Visconti PE, Krapf D. Central role of soluble adenylyl cyclase and cAMP in sperm physiology. Biochim Biophys Acta. 2014;1842(12 Pt B):2610–20. doi: 10.1016/j.bbadis.2014.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rotfeld H, Hillman P, Ickowicz D, Breitbart H. PKA and CaMKII mediate PI3K activation in bovine sperm by inhibition of the PKC/PP1 cascade. Reproduction. 2014;147:347–56. doi: 10.1530/REP-13-0560. [DOI] [PubMed] [Google Scholar]
- 4.Brener E, Rubinstein S, Cohen G, Shternall K, Rivlin J, et al. Remodeling of the actin cytoskeleton during mammalian sperm capacitation and acrosome reaction. Biol Reprod. 2003;68:837–45. doi: 10.1095/biolreprod.102.009233. [DOI] [PubMed] [Google Scholar]
- 5.Breitbart H, Cohen G, Rubinstein S. Role of actin cytoskeleton in mammalian sperm capacitation and the acrosome reaction. Reproduction. 2005;129:263–8. doi: 10.1530/rep.1.00269. [DOI] [PubMed] [Google Scholar]
- 6.Spungin B, Margalit I, Breitbart H. Sperm exocytosis reconstructed in a cell-free system: evidence for the involvement of phospholipase C and actin filaments in membrane fusion. J Cell Sci. 1995;108(Pt 6):2525–35. doi: 10.1242/jcs.108.6.2525. [DOI] [PubMed] [Google Scholar]
- 7.Arnoult C, Kazam IG, Visconti PE, Kopf GS, Villaz M, et al. Control of the low voltage-activated calcium channel of mouse sperm by egg ZP3 and by membrane hyperpolarization during capacitation. Proc Natl Acad Sci U S A. 1999;96:6757–62. doi: 10.1073/pnas.96.12.6757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Itach SB, Finklestein M, Etkovitz N, Breitbart H. Hyper-activated motility in sperm capacitation is mediated by phospholipase D-dependent actin polymerization. Dev Biol. 2012;362:154–61. doi: 10.1016/j.ydbio.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 9.Demott RP, Suarez SS. Hyperactivated sperm progress in the mouse oviduct. Biol Reprod. 1992;46:779–85. doi: 10.1095/biolreprod46.5.779. [DOI] [PubMed] [Google Scholar]
- 10.Katz DF, Vanagimachi R. Movement characteristics of hamster spermatozoa within the oviduct. Biol Reprod. 1980;22:759–64. doi: 10.1095/biolreprod22.4.759. [DOI] [PubMed] [Google Scholar]
- 11.Ho HC, Suarez SS. Hyperactivation of mammalian spermatozoa: function and regulation. Reproduction. 2001;122:519–26. doi: 10.1530/rep.0.1220519. [DOI] [PubMed] [Google Scholar]
- 12.Brown HA, Gutowski S, Moomaw CR, Slaughter C, Sternweis PC. ADP-ribosylation factor, a small GTP-dependent regulatory protein, stimulates phospholipase D activity. Cell. 1993;75:1137–44. doi: 10.1016/0092-8674(93)90323-i. [DOI] [PubMed] [Google Scholar]
- 13.Liscovitch M, Chalifa V, Pertile P, Chen CS, Cantley LC. Novel function of phosphatidylinositol 4,5-bisphosphate as a cofactor for brain membrane phospholipase D. J Biol Chem. 1994;269:21403–6. [PubMed] [Google Scholar]
- 14.Pertile P, Liscovitch M, Chalifa V, Cantley LC. Phosphatidylinositol 4,5-bisphosphate synthesis is required for activation of phospholipase D in U937 cells. J Biol Chem. 1995;270:5130–5. doi: 10.1074/jbc.270.10.5130. [DOI] [PubMed] [Google Scholar]
- 15.Schmidt M, Rümenapp U, Nehls C, Ott S, Keller J, et al. Restoration of Clostridium difficile toxin-B-inhibited phospholipase D by phosphatidylinositol 4,5-bisphosphate. Eur J Biochem. 1996;240:707–12. doi: 10.1111/j.1432-1033.1996.0707h.x. [DOI] [PubMed] [Google Scholar]
- 16.Hodgkin MN, Masson MR, Powner D, Saqib KM, Ponting CP, et al. Phospholipase D regulation and localisation is dependent upon a phosphatidylinositol 4,5-biphosphate-specific PH domain. Curr Biol. 2000;10:43–6. doi: 10.1016/s0960-9822(99)00264-x. [DOI] [PubMed] [Google Scholar]
- 17.Di Paolo G, De Camilli P. Phosphoinositides in cell regulation and membrane dynamics. Nature. 2006;443:651–7. doi: 10.1038/nature05185. [DOI] [PubMed] [Google Scholar]
- 18.Yin HL, Janmey PA. Phosphoinositide regulation of the actin cytoskeleton. Annu Rev Physiol. 2003;65:761–89. doi: 10.1146/annurev.physiol.65.092101.142517. [DOI] [PubMed] [Google Scholar]
- 19.Etkovitz N, Rubinstein S, Daniel L, Breitbart H. Role of PI3-kinase and PI4-kinase in actin polymerization during bovine sperm capacitation. Biol Reprod. 2007;77:263–73. doi: 10.1095/biolreprod.106.056705. [DOI] [PubMed] [Google Scholar]
- 20.Jungnickel MK, Sutton KA, Wang Y, Florman HM. Phosphoinositide-dependent pathways in mouse sperm are regulated by egg ZP3 and drive the acrosome reaction. Dev Biol. 2007;304:116–26. doi: 10.1016/j.ydbio.2006.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Janmey PA, Iida K, Yin HL, Stossel TP. Polyphosphoinositide micelles and polyphosphoinositide-containing vesicles dissociate endogenous gelsolin-actin complexes and promote actin assembly from the fast-growing end of actin filaments blocked by gelsolin. J Biol Chem. 1987;262:12228–36. [PubMed] [Google Scholar]
- 22.Finkelstein M, Etkovitz N, Breitbart H. Role and regulation of sperm gelsolin prior to fertilization. J Biol Chem. 2010;285:39702–9. doi: 10.1074/jbc.M110.170951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Finkelstein M, Megnagi B, Ickowicz D, Breitbart H. Regulation of sperm motility by PIP 2 (4,5) and actin polymerization. Dev Biol. 2013;381:62–72. doi: 10.1016/j.ydbio.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 24.Gremm D, Wegner A. Gelsolin as a calcium-regulated actin filament-capping protein. Eur J Biochem. 2000;267:4339–45. doi: 10.1046/j.1432-1327.2000.01463.x. [DOI] [PubMed] [Google Scholar]
- 25.Yin HL. Gelsolin: calcium- and polyphosphoinositide-regulated actin-modulating protein. Bioessays. 1987;7:176–9. doi: 10.1002/bies.950070409. [DOI] [PubMed] [Google Scholar]
- 26.De Corte V, Demol H, Goethals M, Van Damme J, Gettemans J, et al. Identification of Tyr438 as the major in vitro c-Src phosphorylation site in human gelsolin: a mass spectrometric approach. Protein Sci. 1999;8:234–41. doi: 10.1110/ps.8.1.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kinosian HJ, Newman J, Lincoln B, Selden LA, Gershman LC, et al. Ca2 regulation of gelsolin activity: binding and severing of F-actin. Biophys J. 1998;75:3101–9. doi: 10.1016/S0006-3495(98)77751-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guttman JA, Janmey P, Vogl AW. Gelsolin – evidence for a role in turnover of junction-related actin filaments in Sertoli cells. J Cell Sci. 2002;115(Pt 3):499–505. doi: 10.1242/jcs.115.3.499. [DOI] [PubMed] [Google Scholar]
- 29.Florman HM. Sequential focal and global elevations of sperm intracellular Ca2 are initiated by the zona pellucida during acrosomal exocytosis. Dev Biol. 1994;165:152–64. doi: 10.1006/dbio.1994.1242. [DOI] [PubMed] [Google Scholar]
- 30.Etkovitz N, Tirosh Y, Chazan R, Jaldety Y, Daniel L, et al. Bovine sperm acrosome reaction induced by G-protein-coupled receptor agonists is mediated by epidermal growth factor receptor transactivation. Dev Biol. 2009;334:447–57. doi: 10.1016/j.ydbio.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 31.Varano G, Lombardi A, Cantini G, Forti G, Baldi E, et al. Src activation triggers capacitation and acrosome reaction but not motility in human spermatozoa. Hum Reprod. 2008;23:2652–62. doi: 10.1093/humrep/den314. [DOI] [PubMed] [Google Scholar]
- 32.Baker MA, Hetherington L, Aitken RJ. Identification of SRC as a key PKA-stimulated tyrosine kinase involved in the capacitation-associated hyperactivation of murine spermatozoa. J Cell Sci. 2006;119(Pt 15):3182–92. doi: 10.1242/jcs.03055. [DOI] [PubMed] [Google Scholar]
- 33.Mitchell LA, Nixon B, Baker MA, Aitken RJ. Investigation of the role of SRC in capacitation-associated tyrosine phosphorylation of human spermatozoa. Mol Hum Reprod. 2008;14:235–43. doi: 10.1093/molehr/gan007. [DOI] [PubMed] [Google Scholar]
- 34.Krapf D, Arcelay E, Wertheimer EV, Sanjay A, Pilder SH, et al. Inhibition of Ser/Thr phosphatases induces capacitation-associated signaling in the presence of Src kinase inhibitors. J Biol Chem. 2010;285:7977–85. doi: 10.1074/jbc.M109.085845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lawson C, Goupil S, Leclerc P. Increased activity of the human sperm tyrosine kinase SRC by the cAMP-dependent pathway in the presence of calcium. Biol Reprod. 2008;79:657–66. doi: 10.1095/biolreprod.108.070367. [DOI] [PubMed] [Google Scholar]
- 36.Florman HM, Tombes RM, First NL, Babcock DF. An adhesion-associated agonist from the zona pellucida activates G protein-promoted elevations of internal Ca2 and pH that mediate mammalian sperm acrosomal exocytosis. Dev Biol. 1989;135:133–46. doi: 10.1016/0012-1606(89)90164-4. [DOI] [PubMed] [Google Scholar]
- 37.Jaldety Y, Glick Y, Orr-Urtreger A, Ickowicz D, Gerber D, et al. Sperm epidermal growth factor receptor (EGFR) mediates a7 acetylcholine receptor (AChR) activation to promote fertilization. J Biol Chem. 2012;287:22328–40. doi: 10.1074/jbc.M111.292292. [DOI] [PMC free article] [PubMed] [Google Scholar]


