Fig. 2.
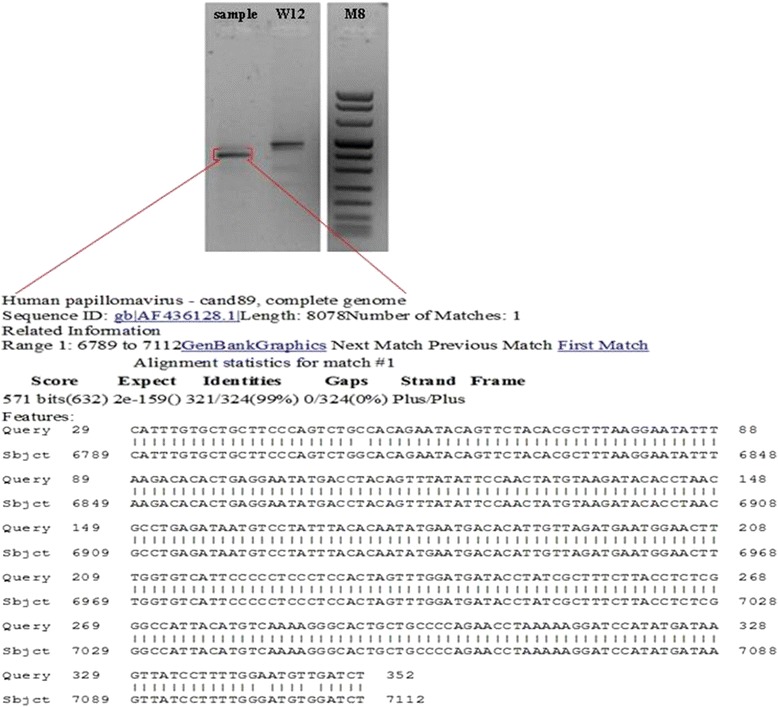
HPV detection and typing. DNA from clinical sample was analysed for HPV detection by PCR with CP degenerate primers that amplify a broad spectrum of HPVs. PCR conditions were 3 mM MgCl2, 200 μM dNTPs, 0.5 μM for each primer and 2.5 U of Platinum TaqDNA polymerase (Life technologies, Milan, Italy) in a final reaction volume of 50 μl. Amplified products were sequenced in an automatic apparatus (Genechron Biogen, Rome, Italy) and sequence analyses were performed using BLAST program (http://www.ncbi.nlm.nih.gov/BLAST). W12 is a cell line containing episomal HPV16 utilized as positive control. M, molecular weight marker VIII (Roche, Milan, Italy)
