Figure 10. Released glycan HPLC-fluorescence-MS analysis and SNA lectin western blotting of IgG produced in ST6GAL1 OE and the control host cell lines.
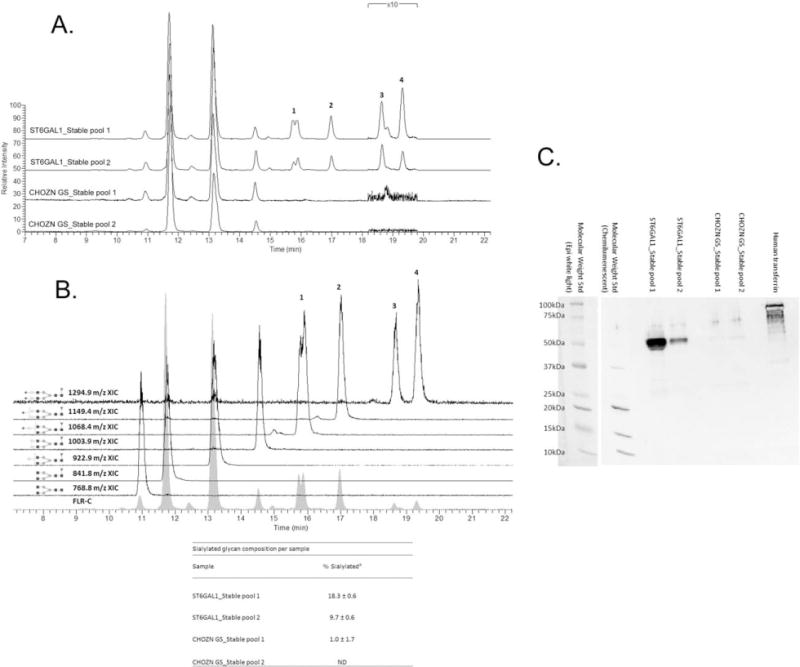
A. Fluorescence HPLC-based glycoprofiles for IgG expressed in transfected and control cell lines. Released N-glycans were labeled with procainamide and resolved by HILIC normal phase chromatography coupled to a fluorescence detector (308 nm excite, 359 nm emission) and the ESI source of a linear trap mass spectrometer. Each feature has been identified by MS as a procainamide-labeled glycan; peaks 1–4 correspond to sialylated glycans. B. Fluorescence and extracted ion chromatograms (XICs) for the glycoprofile of IgG expressed in a ST6Gal1-transfected cell line pool. Each fluorescence peak corresponded to a characteristic doubly charge ion with a mass consistent with the annotated glycan plus procainamide label (+219 Da). The XICs show the elution of these ions values, ±0.5 m/z. Squares are N-acetylglucosamine, grey circles mannose, triangles fucose, light circles galactose, and diamonds N-acetylneuraminic (sialic) acid. Linkage and anomericity is not conveyed by these data. Peaks 1–4 are glycans with atleast one sialic acid residue. The inset table: % sialylated glycan composition based on fluorescence (Mean±SD from three independent cell culture experiments). C. Western blotting using biotinylated SNA. Each lane was loaded with 2 μg purified IgG from one of the three cell culture experiments, and the control lane was loaded with 1 μg human transferrin as the positive control for α2,6-linked sialic acid. The molecular weight standard was imaged with both chemiluminescence and epi-white light due to the short exposure (0.5 and 1 s) required for adequate chemiluminescence imaging of the high-intensity heavy chain bands.
