This article highlights important practice points that impact upon questions of therapy of primary and metastatic gastrointestinal stromal tumor (GIST) to provide examples of what potentially can be achieved with kinase-directed therapies in other cancers. This article also presents cases that highlight some of these key issues in GIST management and addresses both points of consensus and controversial issues in what is now recognized as one of the most common forms of sarcoma.
Keywords: Gastrointestinal stromal tumor, GIST, Imatinib, Sunitinib, Regorafenib, KIT, PDGFRA, SDH, Carney-Stratakis syndrome
Abstract
After the revelation of kinase targeting with orally available small molecules, the use of imatinib in chronic myelogenous leukemia and in gastrointestinal stromal tumor (GIST) has now become commonplace and just two of many examples of the use of kinase inhibitors in cancer. In this article, we discuss important practice points that may impact upon questions of therapy of primary and metastatic GIST, with the hope that the questions addressed in this rare solid tumor can serve as examples of what can be achieved with kinase-directed therapies in other cancers. We present cases that highlight some of the key issues in GIST management and afterward discuss both points of consensus and controversial issues in what is now recognized as one of the most common forms of sarcoma.
Implications for Practice:
The treatment of gastrointestinal stromal tumor (GIST) has become sophisticated with the availability of three approved agents in many countries and 15 years of experience with primary and metastatic disease. Important lessons from tyrosine-kinase inhibitors in GIST can be gleaned from this experience and will impact implementation of similar agents for other cancers.
Introduction
Gastrointestinal stromal tumors (GISTs) are among the most common sarcomas, with an incidence in the range of 10–13 per million per year [1]. What was eventually called GIST was first described as a unique form of cancer in the 1960s, 1970s, and 1980s [2–5]. In 1998, Hirota et al. [6] published the critical paper that irrevocably linked GIST to a mutated gene for a receptor tyrosine kinase gene called KIT and also clearly linked GIST to interstitial cells of Cajal. KIT immunohistochemistry helped reclassifying the disease as GIST. Ultimately, this work ushered in a new wave of studies for this “new” sarcoma subtype.
As was evident in the 1990s and proved in a 2003 clinical trial, GIST is resistant to standard cytotoxic chemotherapy [7]. With the recognition GIST as a KIT-mutated sarcoma, the discovery of KIT inhibitor imatinib as active in GIST cell lines [8] was quickly followed by the response of the first patient with metastatic GIST to imatinib, first treated in March 2000 [9]. The phase I study of imatinib in GIST led to collaborative clinical trials [10–12] and imatinib’s subsequent regulatory approval for metastatic GIST in many countries in 2002. Much has been learned about the management of GIST since the late 1990s. The lessons learned from GIST have become a template for drug development for other kinase inhibitors in a variety of other cancers.
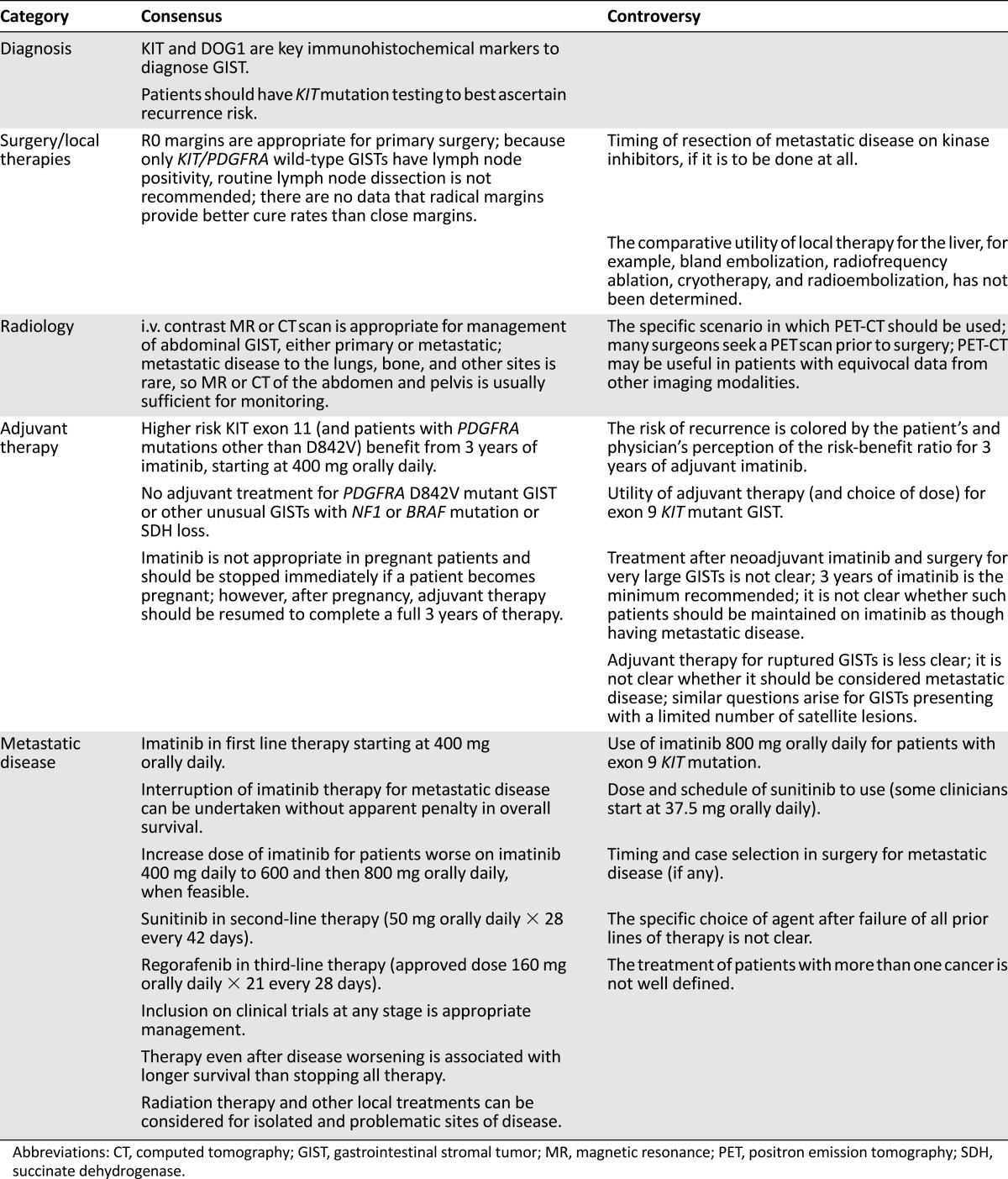
In 2014, we convened to discuss progress in GIST therapy over the prior 15 years, reflecting on patients we have managed. We compiled a dossier of common issues arising in GIST management and developed a vignette-based approach to highlight several of these issues. A table summarizes a variety of key practice issues, not all of which are addressed herein. For details, please refer to national or international guidelines [13–15]. Issues in GIST management are parsed into categories below, specifically (a) primary disease, (b) early metastatic disease, (c) later-stage metastatic disease, and (d) complex cases. We indicate the degree of consensus based on a modification of the National Comprehensive Cancer Network consensus guidelines [16] (Panel 1). For several issues, the best management strategies remain matters of opinion.

Primary Disease
1. A 55-year-old woman presents after resection of a 7-cm gastric GIST. Mutation analysis shows a KIT exon 11 duplication. The tumor shows 1 mitosis per 50 high-power fields. There is no evidence of metastatic disease by imaging.
Would you recommend adjuvant imatinib?
Answer: No
Consensus category: 1B Consensus was not uniform regarding the degree of recurrence risk that merited 3 years of adjuvant therapy.
Discussion
This is a low-risk GIST, and most clinicians would not use 3 years of imatinib in the adjuvant setting [16–18]. The Scandinavian Sarcoma Group (SSG) XVIII study demonstrated benefit of 3 years of adjuvant imatinib in higher-risk GIST [18] (high estimated risk of recurrence, with at least 1 of the following features: (a) greatest tumor diameter more than 10 cm, (b) mitotic count greater than 10 mitoses per 50 high-power fields (hpfs) of the microscope, (c) tumor diameter greater than 5 cm and mitotic count of more than 5 per 50 hpfs, or (d) tumor rupture before surgery or at surgery. In this case, despite its size, the low mitotic rate is the more important factor dictating recurrence risk. From the Joensuu publication on recurrence risk [19] and older publications, this is a lower-risk GIST, and observation alone is the standard of care; for example, only one of 35 such patients from [20] experienced recurrence during follow-up. We acknowledge that the perception of risk varies from patient to patient, and the consensus regarding a plan of action between treatment team and patient is the most important one to achieve.
2. A 27-year-old man presents after resection of a 7-cm small-bowel GIST. The tumor has 8 mitoses in 50 high-powered fields. KIT and PDGFRA are not mutated; subsequent testing reveals loss of expression of succinate dehydrogenase (SDH).
Would you recommend adjuvant imatinib?
Answer: No
Consensus category: 2A
Discussion
By older criteria, this would be considered a high-risk GIST and merit adjuvant imatinib; however, this is a situation in which adjuvant imatinib does not appear to be beneficial. This case highlights what was formerly termed “wild-type” GIST, that is, GIST without a mutation in KIT or PDGFRA.
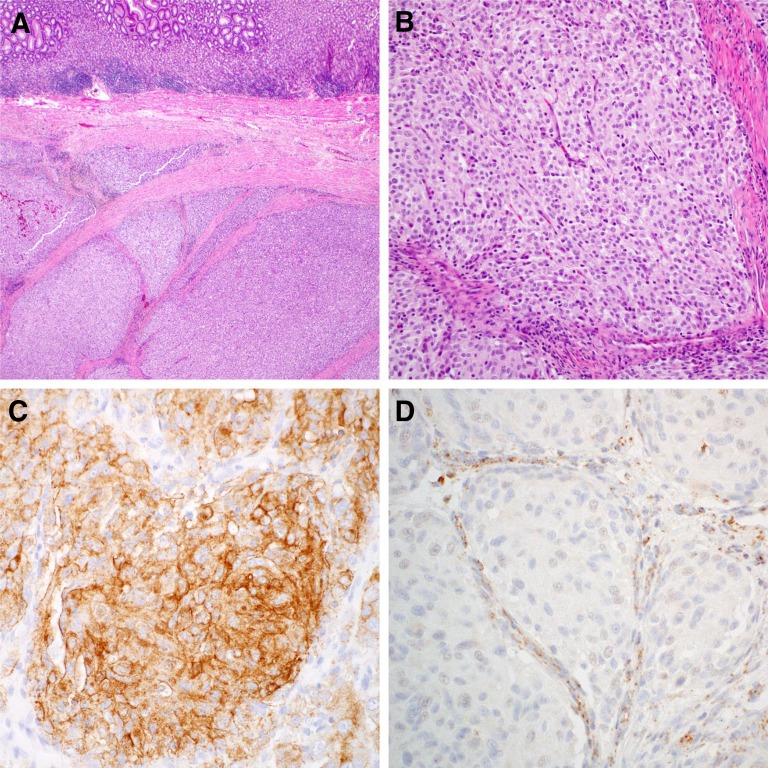
Intensive study of KIT and PDGFRA wild-type GISTs has shown that other genes may be drivers of GIST, including loss of members of the SDH complex [20] (SDHB being most common among these), NF1, or in rare cases other genes such as BRAF. An example of the loss of SDH expression in a KIT and PDGFRA nonmutated GIST is demonstrated in Figure 1. Germline SDH inactivation is also seen in the Carney-Stratakis dyad (paraganglioma and GIST) [21]; this syndrome should be considered in people presenting with SDH-deficient GIST.
Figure 1.
Micrographs of a succinate dehydrogenase (SDH)-deficient gastrointestinal stromal tumor (GIST). Hematoxylin and eosin staining demonstrates typical multinodularity and intervening fibrous septae ([A]: low power; [B]: medium power), KIT immunohistochemistry shows strong KIT expression (C), and SDHB immunohistochemistry shows loss of SDHB expression in the GIST cells, with retained expression in the (noncancerous) fibrous septae as a positive internal control (D).
Analyses by Corless et al. [22] of patients randomized to no adjuvant therapy in the Z9001 American College of Surgeons Oncology Group adjuvant study and by Joensuu et al. [23] of a large cohort of GIST patients not treated with imatinib both showed a low risk of recurrence for certain GISTs, suggesting that imatinib is not indicated for GIST genotypes, such as PDGFRA D842V, and those GISTs without KIT or PDGFRA mutation, both of which have a low risk of recurrence [22]. In addition to the mutation issue, KIT and PDGFRA wild-type GIST that is metastatic does not respond to imatinib but can respond to later generation kinase inhibitors [24, 25]. For these reasons, the authors generally observe patients in this situation or seek out clinical trials.
3. A 62-year-old woman with a resected high-risk GIST of the stomach with a KIT exon 11 mutation has been on adjuvant imatinib for 4 months. She had significant muscle cramping, periorbital and peripheral edema with a 4.2-kg weight gain despite diuretics and a dose reduction to 300 mg daily. Her symptoms from therapy significantly interfere with her activities of daily living, so much so that she would like to stop treatment.
How should you manage this patient?
Answer: Reduce the dose to 200 mg orally daily
Consensus category: 2A
Discussion
Some patients will push on despite toxicity. Some physicians will stop imatinib entirely and follow the patient clinically. However, it is clear in metastatic disease that some patients do not tolerate full-dose imatinib well and still have evidence of benefit at lower doses of imatinib, because of the highly variable clearance of imatinib. In metastatic GIST, imatinib levels were examined to determine whether drug levels are associated with outcomes; patients with plasma levels of drug above 1,110 ng/mL had better outcomes than the quartile of patients below this level [26]. However, no accounting for compliance was undertaken in the study, and studies to examine the role of imatinib level in guiding imatinib dosing failed to accrue. Thus, formal guidance in terms of dose levels remains lacking. In addition, 26% of patients on the 3-year arm of imatinib in the SSG XVIII study discontinued for reasons other than GIST worsening (14% of patients discontinued because of adverse events), and dose reductions were also allowed on study [18]. These data are used to bolster the argument that a low dose is better than no dose in the adjuvant setting in a patient experiencing toxicity. The authors would not hesitate to reduce the dose to 200 mg orally daily to try to complete adjuvant therapy, assuming there were no mitigating circumstances at hand, such as use of medications affecting CYP450 metabolism of imatinib.
4. A 72-year-old man presents with anemia and a palpable left upper quadrant mass. He has a 23-cm tumor in the left upper quadrant by imaging, with no evidence of metastatic disease. He has a biopsy showing GIST, with 22 mitoses in 50 hpfs. Given the difficulty in resection, he starts imatinib with relief of discomfort and shrinking of tumor. After 8 months of therapy, the tumor decreased to 9 cm and showed signs of loss of vascularity consistent with a good response. After appropriate vaccination, he is able to have a small-bowel resection and splenectomy removing the tumor completely (small-bowel primary GIST).
What is your goal for the duration of imatinib therapy?
Answer: 3 years
Consensus category: 2B
Discussion
Patients with this scenario have nearly 100% recurrence without imatinib [19]. Many will recur after 3 years if imatinib is discontinued. However, because some patients will not recur, experts will generally treat for 3 years according to the SSG XVIII study [18] and then watch closely for recurrence with imaging (e.g., computed tomography [CT] abdomen-pelvis every 3–4 months for 2 years following cessation of imatinib); in both the Z9001 and SSG XVIII adjuvant studies the risk of recurrence was highest ∼12 months after discontinuation of adjuvant imatinib [18, 27].
There remains controversy regarding patients presenting with overt tumor rupture, those presenting with a tumor that breaks apart during removal, or those presenting with a limited number of satellite lesions. Should these be considered situations in which “adjuvant” therapy is used, or are these patients with overt stage IV/metastatic disease? The authors argue that there should be a chance to stop imatinib at some point in a patient’s life and that 3 years of imatinib (and close follow-up thereafter) is the most appropriate initial course for systemic therapy. Some clinicians would keep at least a portion of these patients on therapy indefinitely, however. A new study (SSG XXII) will examine 3 versus 5 years of adjuvant imatinib for the highest risk GISTs.
Early Metastatic Disease
5. A patient sees you in follow-up for metastatic GIST; he had 18F-fluorodeoxyglucose positron emission tomography (PET)-CT with diagnostic CT prior to surgery and has been followed this way since diagnosis. He shows evidence of stable disease after 18 months of imatinib.
How would you follow this patient radiologically?
Answer: CT or magnetic resonance imaging (MRI) of abdomen and pelvis with and without IV contrast
Consensus category: 2A
Discussion
Because some patients can be on therapy for a decade or more, consideration should be given to minimizing radiation exposure. GISTs only rarely metastasize to the lungs; intrathoracic disease from GISTs may be most common for GISTs that arise in the esophagus or gastroesophageal junction or in patients refractory to imatinib after several years of therapy, usually only when there are already significant liver and peritoneal sites of disease. PET-CT does not add significantly to the management of patients with metastatic disease, because PET avid disease correlates very well with IV contrast uptake by tumor [28]. Thus, for staging of metastatic disease, CT or MRI scans of the abdomen and pelvis are appropriate for monitoring patients (the lungs are a rare site of metastatic disease and are usually not followed specifically). Situations in which PET-CT is used include at least (a) some patients due for surgical resection, to ensure no obvious site of metastatic disease; (b) tracking the response of neoadjuvant therapy (e.g., in rectal or esophageal GISTs); (c) patients not tolerating IV CT contrast and not tolerating MRI/MRI contrast; (d) selected patients receiving neoadjuvant therapy; and (e) patients in whom tumor worsening is uncertain on standard CT or MRI imaging.
6. A 65-year-old woman with gastric GIST, KIT exon 11 557-558 deletion with 7 mitoses per 50 hpfs, has multifocal metastatic disease develop in the liver and peritoneum 16 months after completion of 3 years of adjuvant imatinib 400 mg orally daily, which she tolerated fairly well, with some evidence of anemia and periorbital edema.
How do you proceed with further therapy?
Answer: Resume imatinib 400 mg orally daily
Consensus category: 1A
Discussion
The authors have seen a number of patients in consultation who have been treated with sunitinib in this situation, with the thought that they had failed imatinib therapy and thus needed a different second-line treatment. However, it is clear from both practical experience as well as studies with metastatic GIST such as BFR14 [29] that most patients remain sensitive to imatinib for a period of time. In this case, imatinib has been more cytostatic than tumoricidal but remains active, much as paclitaxel can be used in rechallenge of patients with breast cancer patients more than 12 months after completion of adjuvant therapy. Because sunitinib is more toxic for most patients and because there are limited options for treatment of metastatic GIST, rechallenge with imatinib is most appropriate in this situation. Sunitinib should be considered after failure of an imatinib rechallenge.
7. A 52-year-old man is seen in follow-up with recent diagnosis of small-bowel GIST metastatic to liver at presentation. He was started on imatinib at 400 mg orally daily 6 weeks ago and is tolerating therapy with periorbital edema and minimal fatigue and feels well. Mutation testing returns showing a KIT exon 9 mutation.
How do you proceed with further therapy?
Answer: Increase total imatinib dose to 800 mg daily
Consensus category: 2A
Discussion
The two large randomized phase III trials of 400 mg of imatinib versus 800 mg of imatinib for metastatic disease showed no difference in overall survival in unselected patients; progression-free survival (PFS) was better in the higher dose arm by approximately 3 months, but because of crossover to a higher dose, survival was not statistically different.
However, in a subset analysis of KIT exon 9 GIST patients, there was improved PFS and a trend to improved survival in patients receiving 800 mg of imatinib daily compared with those receiving 400 mg daily [10, 30]. The toxicity of imatinib in this patient is significantly less than the patient above receiving adjuvant therapy (case 3). As a result, most experts agree that in patients who can tolerate the higher dose, there is a preference to use 800 mg of imatinib daily in exon 9 patients when it can be obtained.
As with lung metastatic disease from other soft tissue sarcoma, the best candidates for surgery for metastatic disease are those who show response or stability and a small number of metastatic foci evident on imaging.
8. A 52-year-old man presents with four peritoneal and two liver metastatic lesions after previously undergoing surgery for an 8-cm GIST affecting the gastric fundus. The tumor had a KIT exon 11 mutation and 6 mitoses in the 50 hpfs analyzed. He is started on imatinib and has stable disease by RECIST after 9 months of therapy.
How do you manage the local and metastatic disease in this patient?
Answer: Discuss surgical resection of metastatic disease; continue imatinib
Consensus category: 2A
Discussion
This question draws on the evidence of benefit seen for surgery for people with metastatic GIST on imatinib who have gone to surgery. Patients in this situation who have surgery or other local therapy such as radiofrequency ablation of liver lesions live longer on average if they have procedures when their metastatic disease is stable or better compared with people with isolated disease progression, who in turn do better than people who have disease progression on imatinib [31–34]. This observation likely represents a lead-time bias, in which the people who are earlier in their course do better than those clinically later in their course, data that were confirmed in a single randomized trial that was not completed, because of low accrual [35]. The counterargument to surgery in this situation is that there are patients who are on imatinib for over 10 years without the need for surgery.
If not a clinical trial candidate, it is reasonable to discuss local therapy; it is clear that imatinib is not curative in the vast majority of patients and that resistant disease remains despite imatinib or other tyrosine-kinase inhibitor therapy; surgery will remove resistant disease before it becomes apparent radiologically. Whether people live longer than they otherwise would is unproved, however. As with lung metastatic disease from other soft tissue sarcoma, the best candidates for surgery for metastatic disease are those who show response or stability and a small number of metastatic foci evident on imaging. We note that it is common practice to resume the previous tyrosine-kinase inhibitor after healing from surgery is complete.
Later Stage Metastatic Disease
9. You follow a 63-year-old woman with metastatic gastric GIST to the peritoneum and liver (exon 11 KIT mutant) who has been treated for 11 years with sustained partial response of disease with resolution of peritoneal metastatic disease and evidence of low density liver metastases that have slowly decreased in size. She has had persistent grade 1 periorbital edema and still cramping episodes most notable in the hands and feet once to twice a week. She is interested in a break from treatment.
What do you advise her?
Answer: Agree to a 6–12 month hiatus from imatinib with reimaging every 3 months
Consensus category: 2B
Discussion
As many as 15%–20% of people are able to remain on imatinib for 10 years or longer. Given evidence of continued disease worsening in other patients between years 5 and 10 of treatment, it is difficult to believe that such patients are cured of disease. The most common genotype the authors have seen associated with long-term survival are exon 11 KIT mutations, in particular those who had a low mitotic rate. The data from the BFR14 study indicate that people may stop imatinib for a period of time without a penalty in overall survival [29, 36]. With the understanding that there is at least some risk of resistant disease after stopping and restarting imatinib, a break from therapy is a reasonable option for people with metastatic GIST with good results with imatinib for several years. As with high risk metastatic disease, disease worsening will be evident in most people within the first year after treatment discontinuation. As a trade-off, if treatment is interrupted, it is logical to increase the frequency of restaging scans, in an attempt to identify recurrence before patients are overtly symptomatic.
With the understanding that there is at least some risk of resistant disease after stopping and restarting imatinib, a break from therapy is a reasonable option for people with metastatic GIST with good results with imatinib for several years.
10. A 52-year-old man with metastatic KIT exon 9 GIST has gotten worse after 14 months of imatinib, 9 months on sunitinib, and 6 months on regorafenib. He has large-volume liver and peritoneal metastatic disease. He is still able to perform his usual activities of daily living. He has hypertension controlled by medication and stable grade 1 skin changes on his palms and soles on regorafenib.
How do you suggest further managing the patient?
Answer: Continue a tyrosine-kinase inhibitor
Consensus category: 2A
Discussion
After failure of imatinib for metastatic disease, sunitinib and regorafenib have been approved widely for use in refractory metastatic GIST [37, 38]. For patients failing existing lines of systemic therapy, enrollment on a clinical trial is preferred, but not an option available to everyone. In one randomized trial, a clinical suspicion was confirmed: people who are kept on therapy, in this case with resumption of imatinib, lived longer than people who received no further tyrosine-kinase inhibitor therapy [39]. Thus, disease worsening appears to be slowed with treatment compared with stopping treatment entirely. For people tolerating their most recent line of therapy, it is reasonable to continue that most recent line of therapy. Radiation therapy may be used in people with anatomically favorable sites of disease that are worsening.
Complex Cases
11. A 35-year-old woman has just completed 1 year of adjuvant imatinib for a small-bowel GIST, 7 cm, with 3 mitoses per 50 hpfs. The tumor has an exon 11 KIT deletion. She is interested in having children and asks you if she can stop her imatinib so that she can try to get pregnant.
What do you advise her?
Answer: Hold imatinib for up to 12 months, counseling on the possibility of decreased cure rate and metastatic disease while pregnant or after pregnancy; reimage (bearing in mind possible pregnancy) quarterly
Consensus category: 3
Discussion
This is a personal decision that will require balancing the possible benefit of imatinib and risk of recurrence and death from GIST against her desire to have a child or children. As an oncologist, you can bring a discussion of risk to the table and the ability to discuss complications if she has a recurrence when she is pregnant or after parturition. In this particular scenario, she has a ∼50% chance of recurrence of her GIST. In the authors’ experience, imatinib can interfere to some degree with menses, with the perception of a somewhat higher rate of menorrhagia. As a result, her ability to conceive after stopping imatinib should be close to normal within a few months of stopping imatinib. Metastatic GIST has not been known to affect the uterus or ovaries; however, given prior surgery, her risk of ectopic pregnancy is higher. If she opts for other than normal means of conception, for example, using in vitro fertilization, without massive tumor burden, she would be expected to be able to carry to full term. The BFR14 data also indicate that most people will retain imatinib sensitivity despite stopping imatinib. All of these factors will figure in the decision that one can mutually agree upon for such a patient. We note that women who reach full term for their pregnancies are generally restarted on adjuvant therapy, to ensure the mother has the greatest chance of cure.
12. You have followed a 62-year-old woman with metastatic gastric GIST to peritoneum and liver with very good partial response to treatment over the past 6 years. She palpates a left breast mass and has this biopsied, demonstrating an ER+ HER2+ breast cancer. She has surgery and is seen to have a 3.5-cm primary tumor and two positive sentinel lymph nodes.
How do you manage both cancer diagnoses in this patient?
Answer: Interrupt imatinib to administer adjuvant chemotherapy for breast cancer; resume imatinib during estrogen deprivation
Consensus category: 3
Discussion
Imatinib has been successful enough for metastatic GIST that common things continue to occur, for example, coronary artery disease, flares of chronic obstructive pulmonary disease, or second cancers. The authors have had a number of patients with new primary cancers diagnosed while on imatinib for metastatic GIST, and it is not surprising that these include common diagnoses such as breast and prostate cancer. It is thus clear that imatinib does not prevent breast or prostate cancer, but it is also clear that treatment for the second cancer can be undertaken if the risk profile is significant enough for metastatic disease.
The authors note that hormonal therapy, for example, tamoxifen and aromatase inhibitors, can be administered concomitantly with imatinib. The risk of the breast cancer would then dictate the utility of chemotherapy or not. For example, in ER+ HER2+ breast cancer patients, patients often are recommended both cytotoxic and HER2-directed therapy. The BFR14 data prove very useful here—it is possible to interrupt imatinib without penalty in overall survival for people with metastatic GIST on imatinib. Chemotherapy can be given in an imatinib-free interval and started after completion of dose-dense systemic chemotherapy, for example, traditional doxorubicin-cyclophosphamide followed by paclitaxel in addition to the trastuzumab, with monitoring for worsening of the metastatic GIST. The authors have seen patients treated anecdotally with concurrent imatinib and cytotoxic chemotherapy, which appears to be significantly more toxic than the chemotherapy itself in the patients the authors have seen. A final unknown here is the pharmacokinetic/pharmacodynamic interaction of chemotherapy or hormonal therapy with imatinib or other tyrosine-kinase inhibitors.
Conclusion
Having completed the questions above, in Table 1, we summarize consensus and controversial points in GIST management, some of which are not discussed in the text. It is not surprising that consensus on a given topic is largely correlated to the quality of the available data. It is notable that when similar studies have been done in more than one setting, the data are consistent, perhaps reflecting the relatively simple genomics of GIST. Although new agents are under development for GIST, it is important to use the limited tools we have to our patients’ greatest benefit. The authors hope that these scenarios will help sharpen practitioners’ acumen regarding treatment of this common form of sarcoma and that this experience can guide physicians studying kinase inhibitors in other diseases.
Table 1.
Consensus and controversial points in GIST management
Acknowledgment
Bayer Pharmaceuticals provided support for a meeting that led to the concept of this article. The authors are solely responsible for the content and have received no support from outside sources for its writing or preparation.
Author Contributions
Conception/Design: Robert G. Maki, Jean-Yves Blay, George D. Demetri, Jonathan A. Fletcher, Heikki Joensuu, Javier Martín-Broto, Toshirou Nishida, Peter Reichardt, Patrick Schöffski, Jonathan C. Trent
Provision of study material or patients: Jean-Yves Blay, George D. Demetri, Jonathan A. Fletcher, Heikki Joensuu, Javier Martín-Broto, Toshirou Nishida, Peter Reichardt, Patrick Schöffski, Jonathan C. Trent
Collection and/or assembly of data: Robert G. Maki, Jean-Yves Blay, George D. Demetri, Jonathan A. Fletcher, Heikki Joensuu, Javier Martín-Broto, Toshirou Nishida, Peter Reichardt, Patrick Schöffski, Jonathan C. Trent
Data analysis and interpretation: Robert G. Maki, Jean-Yves Blay, George D. Demetri, Jonathan A. Fletcher, Heikki Joensuu, Javier Martín-Broto, Toshirou Nishida, Peter Reichardt, Patrick Schöffski, Jonathan C. Trent
Manuscript writing: Robert G. Maki, Jean-Yves Blay, George D. Demetri, Jonathan A. Fletcher, Heikki Joensuu, Javier Martín-Broto, Toshirou Nishida, Peter Reichardt, Patrick Schöffski, Jonathan C. Trent
Final approval of manuscript: Robert G. Maki, Jean-Yves Blay, George D. Demetri, Jonathan A. Fletcher, Heikki Joensuu, Javier Martín-Broto, Toshirou Nishida, Peter Reichardt, Patrick Schöffski, Jonathan C. Trent
Disclosures
Robert G. Maki: Bayer (C/A, H), Eisai (C/A, H, RF), Tracon (C/A, RF), Lilly (H, RF), Sarcoma Alliance for Research through Collaboration (participation/leadership); Jean-Yves Blay: Novartis, Pfizer, Bayer (C/A, RF); George D. Demetri: Blueprint Medicines Board of Directors (C/A, E, OI), Dana-Farber (IP), Novartis (C/A, IP, RF), Pfizer, Bayer, GlaxoSmithKline (C/A, RF), Ariad, AstraZeneca (C/A), Kolltan (C/A, OI); Jonathan A. Fletcher: Bayer, Ariad (C/A); Heikki Joensuu: Blueprint Medicines (RF); Javier Martín-Broto: PharmaMar, GlaxoSmithKline, Bayer, Amgen (C/A); Toshirou Nishida: Bayer, Pfizer (H); Peter Reichardt: Pfizer, Bayer (C/A, H), Ariad (C/A), Novartis (RF); Patrick Schöffski: Novartis (C/A, RF), Bayer (C/A). The other author indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Goettsch WG, Bos SD, Breekveldt-Postma N, et al. Incidence of gastrointestinal stromal tumours is underestimated: Results of a nation-wide study. Eur J Cancer. 2005;41:2868–2872. doi: 10.1016/j.ejca.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 2.Martin JF, Bazin P, Feroldi J, et al. [Intramural myoid tumors of the stomach. Microscopic considerations on 6 cases] Ann Anat Pathol (Paris) 1960;5:484–497. [PubMed] [Google Scholar]
- 3.Stout AP. Bizarre smooth muscle tumors of the stomach. Cancer. 1962;15:400–409. doi: 10.1002/1097-0142(196203/04)15:2<400::aid-cncr2820150224>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 4.Appleman HD, Helwig EB. Gastric epithelioid leiomyoma and leiomyosarcoma (leiomyoblastoma) Cancer. 1976;38:708–728. doi: 10.1002/1097-0142(197608)38:2<708::aid-cncr2820380215>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 5.Mazur MT, Clark HB. Gastric stromal tumors: Reappraisal of histogenesis. Am J Surg Pathol. 1983;7:507–519. doi: 10.1097/00000478-198309000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Hirota S, Isozaki K, Moriyama Y, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279:577–580. doi: 10.1126/science.279.5350.577. [DOI] [PubMed] [Google Scholar]
- 7.Trent JC, Beach J, Burgess MA, et al. A two-arm phase II study of temozolomide in patients with advanced gastrointestinal stromal tumors and other soft tissue sarcomas. Cancer. 2003;98:2693–2699. doi: 10.1002/cncr.11875. [DOI] [PubMed] [Google Scholar]
- 8.Tuveson DA, Willis NA, Jacks T, et al. STI571 inactivation of the gastrointestinal stromal tumor c-KIT oncoprotein: Biological and clinical implications. Oncogene. 2001;20:5054–5058. doi: 10.1038/sj.onc.1204704. [DOI] [PubMed] [Google Scholar]
- 9.Joensuu H, Roberts PJ, Sarlomo-Rikala M, et al. Effect of the tyrosine kinase inhibitor STI571 in a patient with a metastatic gastrointestinal stromal tumor. N Engl J Med. 2001;344:1052–1056. doi: 10.1056/NEJM200104053441404. [DOI] [PubMed] [Google Scholar]
- 10.Verweij J, Casali PG, Zalcberg J, et al. Progression-free survival in gastrointestinal stromal tumours with high-dose imatinib: Randomised trial. Lancet. 2004;364:1127–1134. doi: 10.1016/S0140-6736(04)17098-0. [DOI] [PubMed] [Google Scholar]
- 11.Demetri GD, von Mehren M, Blanke CD, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med. 2002;347:472–480. doi: 10.1056/NEJMoa020461. [DOI] [PubMed] [Google Scholar]
- 12.Blanke CD, Rankin C, Demetri GD, et al. Phase III randomized, intergroup trial assessing imatinib mesylate at two dose levels in patients with unresectable or metastatic gastrointestinal stromal tumors expressing the kit receptor tyrosine kinase: S0033. J Clin Oncol. 2008;26:626–632. doi: 10.1200/JCO.2007.13.4452. [DOI] [PubMed] [Google Scholar]
- 13.ESMO/European Sarcoma Network Working Group Gastrointestinal stromal tumours: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25(suppl 3):iii21–iii26. doi: 10.1093/annonc/mdu255. [DOI] [PubMed] [Google Scholar]
- 14.von Mehren M, Randall RL, Benjamin RS, et al. Gastrointestinal stromal tumors, version 2.2014. J Natl Compr Canc Netw. 2014;12:853–862. doi: 10.6004/jnccn.2014.0080. [DOI] [PubMed] [Google Scholar]
- 15.Rubin BP, Blanke CD, Demetri GD, et al. Protocol for the examination of specimens from patients with gastrointestinal stromal tumor. Arch Pathol Lab Med. 2010;134:165–170. doi: 10.5858/134.2.165. [DOI] [PubMed] [Google Scholar]
- 16.von Mehren M, Randall RL, Benjamin RS et al. Soft tissue sarcoma, version 01.2015. Available at http://www.nccn.org. Accessed March 1, 2015.
- 17.DeMatteo RP, Ballman KV, Antonescu CR, et al. Long-term results of adjuvant imatinib mesylate in localized, high-risk, primary gastrointestinal stromal tumor: ACOSOG Z9000 (Alliance) intergroup phase 2 trial. Ann Surg. 2013;258:422–429. doi: 10.1097/SLA.0b013e3182a15eb7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Joensuu H, Eriksson M, Sundby Hall K, et al. One vs three years of adjuvant imatinib for operable gastrointestinal stromal tumor: A randomized trial. JAMA. 2012;307:1265–1272. doi: 10.1001/jama.2012.347. [DOI] [PubMed] [Google Scholar]
- 19.Joensuu H, Vehtari A, Riihimäki J, et al. Risk of recurrence of gastrointestinal stromal tumour after surgery: An analysis of pooled population-based cohorts. Lancet Oncol. 2012;13:265–274. doi: 10.1016/S1470-2045(11)70299-6. [DOI] [PubMed] [Google Scholar]
- 20.Janeway KA, Kim SY, Lodish M, et al. Defects in succinate dehydrogenase in gastrointestinal stromal tumors lacking KIT and PDGFRA mutations. Proc Natl Acad Sci USA. 2011;108:314–318. doi: 10.1073/pnas.1009199108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perry CG, Young WF, Jr, McWhinney SR, et al. Functioning paraganglioma and gastrointestinal stromal tumor of the jejunum in three women: Syndrome or coincidence. Am J Surg Pathol. 2006;30:42–49. doi: 10.1097/01.pas.0000178087.69394.9f. [DOI] [PubMed] [Google Scholar]
- 22.Corless CL, Ballman KV, Antonescu CR, et al. Pathologic and molecular features correlate with long-term outcome after adjuvant therapy of resected primary GI stromal tumor: The ACOSOG Z9001 trial. J Clin Oncol. 2014;32:1563–1570. doi: 10.1200/JCO.2013.51.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Joensuu H, Rutkowski P, Nishida T, et al. KIT and PDGFRA mutations and the risk of GI stromal tumor recurrence. J Clin Oncol. 2015;33:634–642. doi: 10.1200/JCO.2014.57.4970. [DOI] [PubMed] [Google Scholar]
- 24.Janeway KA, Albritton KH, Van Den Abbeele AD, et al. Sunitinib treatment in pediatric patients with advanced GIST following failure of imatinib. Pediatr Blood Cancer. 2009;52:767–771. doi: 10.1002/pbc.21909. [DOI] [PubMed] [Google Scholar]
- 25.Ganjoo KN, Villalobos VM, Kamaya A, et al. A multicenter phase II study of pazopanib in patients with advanced gastrointestinal stromal tumors (GIST) following failure of at least imatinib and sunitinib. Ann Oncol. 2014;25:236–240. doi: 10.1093/annonc/mdt484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Demetri GD, Wang Y, Wehrle E, et al. Imatinib plasma levels are correlated with clinical benefit in patients with unresectable/metastatic gastrointestinal stromal tumors. J Clin Oncol. 2009;27:3141–3147. doi: 10.1200/JCO.2008.20.4818. [DOI] [PubMed] [Google Scholar]
- 27.Dematteo RP, Ballman KV, Antonescu CR, et al. Adjuvant imatinib mesylate after resection of localised, primary gastrointestinal stromal tumour: A randomised, double-blind, placebo-controlled trial. Lancet. 2009;373:1097–1104. doi: 10.1016/S0140-6736(09)60500-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Choi H, Charnsangavej C, Faria SC, et al. Correlation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate: Proposal of new computed tomography response criteria. J Clin Oncol. 2007;25:1753–1759. doi: 10.1200/JCO.2006.07.3049. [DOI] [PubMed] [Google Scholar]
- 29.Patrikidou A, Chabaud S, Ray-Coquard I, et al. Influence of imatinib interruption and rechallenge on the residual disease in patients with advanced GIST: Results of the BFR14 prospective French Sarcoma Group randomised, phase III trial. Ann Oncol. 2013;24:1087–1093. doi: 10.1093/annonc/mds587. [DOI] [PubMed] [Google Scholar]
- 30.Debiec-Rychter M, Sciot R, Le Cesne A, et al. KIT mutations and dose selection for imatinib in patients with advanced gastrointestinal stromal tumours. Eur J Cancer. 2006;42:1093–1103. doi: 10.1016/j.ejca.2006.01.030. [DOI] [PubMed] [Google Scholar]
- 31.Bauer S, Hartmann JT, de Wit M, et al. Resection of residual disease in patients with metastatic gastrointestinal stromal tumors responding to treatment with imatinib. Int J Cancer. 2005;117:316–325. doi: 10.1002/ijc.21164. [DOI] [PubMed] [Google Scholar]
- 32.Gold JS, Dematteo RP. Combined surgical and molecular therapy: The gastrointestinal stromal tumor model. Ann Surg. 2006;244:176–184. doi: 10.1097/01.sla.0000218080.94145.cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rutkowski P, Nowecki Z, Nyckowski P, et al. Surgical treatment of patients with initially inoperable and/or metastatic gastrointestinal stromal tumors (GIST) during therapy with imatinib mesylate. J Surg Oncol. 2006;93:304–311. doi: 10.1002/jso.20466. [DOI] [PubMed] [Google Scholar]
- 34.Hohenberger P, Eisenberg B. Role of surgery combined with kinase inhibition in the management of gastrointestinal stromal tumor (GIST) Ann Surg Oncol. 2010;17:2585–2600. doi: 10.1245/s10434-010-1053-9. [DOI] [PubMed] [Google Scholar]
- 35.Du CY, Zhou Y, Song C, et al. Is there a role of surgery in patients with recurrent or metastatic gastrointestinal stromal tumours responding to imatinib: A prospective randomised trial in China. Eur J Cancer. 2014;50:1772–1778. doi: 10.1016/j.ejca.2014.03.280. [DOI] [PubMed] [Google Scholar]
- 36.Blay JY, Pérol D, Le Cesne A. Imatinib rechallenge in patients with advanced gastrointestinal stromal tumors. Ann Oncol. 2012;23:1659–1665. doi: 10.1093/annonc/mdr622. [DOI] [PubMed] [Google Scholar]
- 37.Demetri GD, van Oosterom AT, Garrett CR, et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: A randomised controlled trial. Lancet. 2006;368:1329–1338. doi: 10.1016/S0140-6736(06)69446-4. [DOI] [PubMed] [Google Scholar]
- 38.Demetri GD, Reichardt P, Kang YK, et al. Efficacy and safety of regorafenib for advanced gastrointestinal stromal tumours after failure of imatinib and sunitinib (GRID): An international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381:295–302. doi: 10.1016/S0140-6736(12)61857-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kang YK, Ryu MH, Yoo C, et al. Resumption of imatinib to control metastatic or unresectable gastrointestinal stromal tumours after failure of imatinib and sunitinib (RIGHT): A randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2013;14:1175–1182. doi: 10.1016/S1470-2045(13)70453-4. [DOI] [PMC free article] [PubMed] [Google Scholar]