Abstract
Perineural invasion is a symbiotic relationship between cancer cells and nerves and is most frequently seen in “neurotropic” cancers, such as prostate cancer. It results in increased perineural space cancer cell growth and decreased apoptosis and induces nerve growth. Tissue microarrays (TMA) were constructed from 640 radical prostatectomy specimens with prostate cancer. The perineural diameter was measured as previously described. Multiple biomarkers have been previously performed on this TMA cohort and all data was kept in the same database. The biomarker results database was queried for correlations between perineural invasion diameter and tissue biomarkers. Increased perineural invasion diameter correlated with increased proliferation of prostate cancer cells, and with apoptosis. It also correlated with proteins involved in survival pathways such as NFkB, C-MYC, phosphorylated AKT and its downstream effector FHKR, but not with GSK. Unlike nerve density it did not correlate with decreased PTEN expression. Increased perineural invasion diameter was associated with higher levels of hormonal receptors such as androgen receptor, but not estrogen receptor. Also associated with perineural invasion diameter were co-regulators and co-repressors including SRC1 and TIF2. Perineural invasion diameter had the strongest correlation with tumor volume (rho=0.579; p=0.000), not identified with nerve density. These data demonstrate that perineural invasion has similar biologic correlations as neural density. However, we found a distinct and very strong correlation with increased tumor volume. This data confirms that perineural invasion is the ultimate and most successful interaction between cancer cells and nerve fibers, resulting in increased tumor growth.
Keywords: Perineural invasion diameter, prostate cancer
INTRODUCTION
Perineural invasion (PNI), seen most frequently in “neurotropic” cancers, is a common finding in prostate cancer (PCa). Prostatic adenocarcinoma penetrates the prostatic capsule and it is one of the mechanisms by which PCa extends beyond the capsule (1). During the last decade much progress has been made to understand the biology of PNI (2). Understanding this biology has helped us to better understand the microscopic neuroanatomy of the prostate (3) and has also revealed a symbiotic relationship between cancer cells and nerves. Therefore, modifying the aggressive behavior of PCa resides in understanding the significance and regulatory processes of perineural invasion.
We have previously shown, using an in vitro model that PNI was an active, specific, and reciprocal manner of interaction between nerves and PCa cells (2). PNI resulted in enhanced proliferation and reduced apoptosis in culture with nerves and in human tissues. These cancer cells in the perineural space hence acquire a survival advantage (4–6). We have demonstrated that caveolin secretion by the nerves and NFkB activation in the cancer cells are involved in the survival advantage of cancer cells (7). In the process of PNI, cancer cells use bystin, an embryonic attachment protein (8). These phenomena also translate to human tissues.
In addition, the symbiotic relationship results in cancer-induced stimulus for nerve growth. We have described this novel biologic phenomenon as cancer-related axonogenesis and neurogenesis, as not only is the nerve density higher in cancer foci, but the number of neurons in periprostatic ganglia of cancer patients is higher than in normal controls (9). Axonogenesis can be detected in preneoplastic lesions.
PNI has historically been a significant biomarker in PCa. In biopsies it is a predictor of extracapsular extension (10). In radical prostatectomy (RP) specimens the presence of PNI is not predictive of survival (11). This is probably due to the fact that PNI is a common occurrence in prostate cancer. There is however a biomarker that measures the success of the interaction between nerves and cancer, PNI diameter, and it is highly predictive of biochemical recurrence. Maru et al showed that the maximum diameter of PNI by cancer in RP specimens was strongly associated with adverse pathologic features and was an independent predictor of biochemical free recurrence (12). Biologically the maximum diameter of cancer along the nerve is associated with a successful interaction between nerves and cancer cells that results in increased cancer growth around the nerve. Therefore, the PNI diameter becomes a surrogate marker for the successful interaction between cancer cells and nerves. As such, its predictive ability, as a measure of successful biology, is significant.
The objective of this study is to examine the biologic significance of PNI in human PCa by correlating PNI diameter to biomarkers in our dataset, previously performed on the same cohort of patients, expressed in cancer cells in the perineural space and away from PNI. We will show the influence that nerve fibers, through PNI, have on cancer cells.
MATERIALS AND METHODS
Clinical and pathologic characteristics
The initial cohort consisted of 1210 patients who underwent RP at Baylor College of Medicine (Houston, TX)–affiliated hospitals between 1983 and 1998. We qualified 640 cases for building TMAs based on the following criteria: (1) patients did not receive preoperative treatment, (2) patients had surgery between 1983 and 1998, and (3) sufficient PCa tissue was available for building TMAs. The full patient characteristics cohort has been published before (13, 14). For this study 637 patients had available PNI diameter measurements. The characteristics of this cohort have been previously published (12).
Statistical analysis
PNI diameter expression was analyzed for correlations (Spearman’s test) with tissue biomarkers already present in our database. All analyses were performed with the SPSS 11.0 (SPSS, Chicago, IL) statistical software.
RESULTS
Correlations between PNI diameter and PCa biomarker expression in our database
PNI diameter correlations with clinico-pathologic parameters were previously published (12). PNI diameter (Figure 1) was now correlated with tissue biomarkers available in our database.
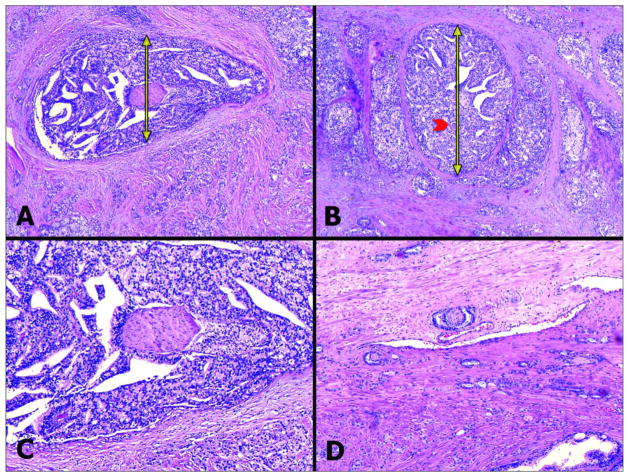
Figure 1.
A, B - Examples of PNI with very large PNI diameter. The PNI diameter’s value is established by measuring the tumor diameter (yellow double arrows) around the nerve (red arrow head) perpendicular to the large axis of the nerve (H&E 20X). C – Higher magnification (40X) of A. D - For comparison purposes, this image (H&E, 40X) shows PNI with low PNI diameter.
Most biomarkers that shared correlations with nerve density, presented in the companion paper, shared similar correlations with PNI diameter. These included proteins implicated in survival pathways, hormone response elements, co-regulators, and repressors. Selected values are represented in Tables 1 and 2 and Figure 2. Increased PNI diameter correlated with increased proliferation of PCa cells, as measured by Ki-67 (rho=0.167; p=0.0015). Surprisingly we also found a non-expected correlation with apoptosis, as measured by Tunel (rho=0.157; p=0.0049). It also correlated with expression of proteins involved in survival pathways such as phosphorylated AKT (rho=0.140; p=0.0059) and downstream effectors such as the phosphorylated nuclear FHKR (rho=0.174; p=0.0029) but not with phosphorylated GSK (rho=0.075; p=0.1161). Increased PNI diameter was not significantly associated with decreased PTEN expression (rho=−0.090; p=0.1429), unlike nerve density (rho=−0.178; p=0.0356). Activation of the NFkB pathway was confirmed by positive correlation with the nuclear expression of NFkB (rho=0.139; p=0.0043). However, cytoplasmic PIM-2, a downstream effector of NFkB was not associated with increased PNI diameter (rho=0.034; p=0.5420). The downstream effector C-MYC was positively associated (C-MYC Cytoplasmic – rho=0.111, p=0.0564; C-MYC Nuclear - rho=0.118; p=0.0413).
Table 1.
Correlations between PNI diameter and biomarkers expressed by the prostate cancer cells.
| Biomarker | Rho | p-value |
|---|---|---|
| Androgen receptor | 0.123 | 0.0143 |
| C-Myc Cytoplasmic | 0.111 | 0.0564 |
| C-Myc Nuclear | 0.118 | 0.0413 |
| COUP - II Epithelial | 0.221 | 0.0001 |
| Cyclin D1 Cytoplasmic | 0.14 | 0.012 |
| Cyclin D1 Nuclear | 0.134 | 0.016 |
| GSK2 Cytoplasmic | 0.104 | 0.0582 |
| Index tumor volume | 0.579 | 0 |
| Ki-67 | 0.167 | 0.0015 |
| NFKB 2mm | 0.22 | 0 |
| NFKB Nuclear | 0.139 | 0.0043 |
| Phospho-AKT | 0.14 | 0.0059 |
| Phospho-FHKR Nuclear | 0.174 | 0.0029 |
| PIM2 Nuclear | 0.106 | 0.0587 |
| RTVP Nuclear | 0.117 | 0.0397 |
| SKP2 | 0.158 | 0.0019 |
| SRC1 | 0.131 | 0.0142 |
| TIF2 | 0.113 | 0.0333 |
| Tunel | 0.157 | 0.0049 |
Table 2.
Biomarkers expressed by prostate cancer cells that showed no correlation with PNI diameter.
| Biomarker | Rho | p-value |
|---|---|---|
| AKT22 Nuclear | 0.094 | 0.0796 |
| ER-Alpha quantitation | 0.004 | 0.9458 |
| FHKR Cytoplasmic | 0.089 | 0.1568 |
| FHKR Nuclear | 0.096 | 0.1285 |
| GSK2 Nuclear | 0.063 | 0.2543 |
| NCAM groups | 0.038 | 0.7327 |
| Phospho-FHKR Cytoplasmic | 0.061 | 0.3048 |
| Phospho-GSK | 0.075 | 0.1161 |
| P27 | 0.031 | 0.5347 |
| p53 Cytoplasmic | 0.063 | 0.4642 |
| p53 Nuclear | 0.052 | 0.5427 |
| PA28 Gamma Cytoplasmic | 0.06 | 0.2786 |
| PA28 Gamma Nuclear | 0.028 | 0.608 |
| PGP 9.5 | 0.097 | 0.1147 |
| PIM2 Cytoplasmic | 0.034 | 0.542 |
| PKC Cytoplasmic | 0.016 | 0.8441 |
| PKC Nuclear | 0.036 | 0.6598 |
| PTEN | −0.09 | 0.1429 |
| RTVP Cytoplasmic | −0.002 | 0.9752 |
| SRC3 | 0.059 | 0.4845 |
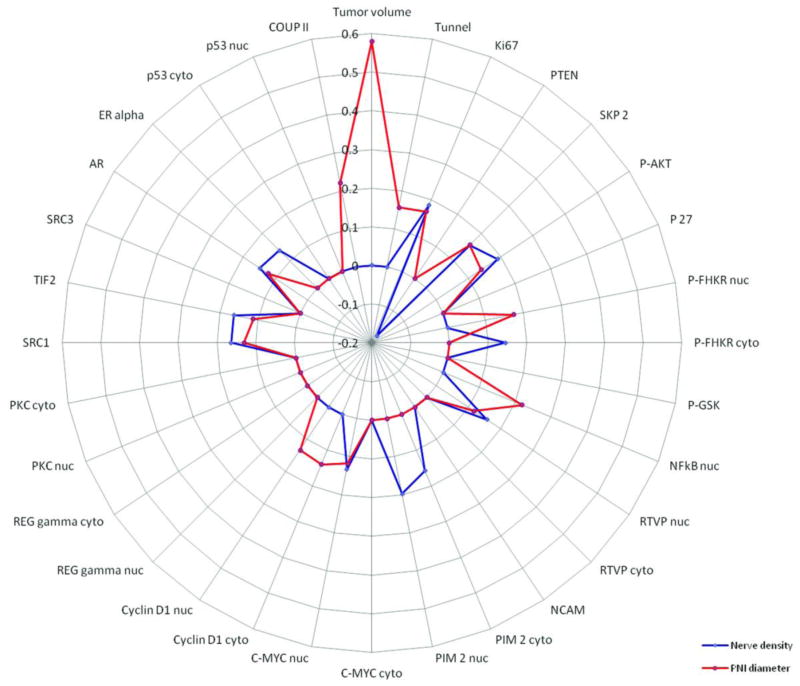
Figure 2.
Visualization of the correlation between biomarkers previously stained in this cohort and nerve density as measured by PGP 9.5 immunoexpression (blue line) and PNI diameter (red line). Absence of correlation is plotted as a dot on the 0 (zero) line, positive correlations are plotted outwards, while negative correlations are plotted inwards, towards the center. The correlation coefficients are labeled at approximately 12:00 o’clock.
Unexpectedly, PNI diameter was associated with hormonal regulation of PCa. Increased PNI diameter was associated with higher levels of androgen hormone receptor expression (rho=0.123; p=0.0143). Unlike neural density, estrogen receptor was not correlated with increased PNI diameter (rho=0.004; p=0.9458). Also associated with PNI diameter were co-regulators and repressors such as SRC1 (rho=0.131; p=0.0142) and SRC2 (TIF2) (rho=0.113; p=0.0333).
PNI diameter had a very strong correlation with tumor volume (rho=0.579; p=0.0000) as opposed to nerve density (rho=−0.020; p=0.7260). This correlation has been previously presented in Maru et al{Maru, 2001 #1425}. However, this cohort does not include all patients in the previous study. The correlation coefficient in this study is specific to the cohort used for this study.”
DISCUSSION
Nerves are a critical component of the tumor microenvironment. They interact with cancer cells in numerous stages of carcinogenesis, starting with cancer induced axonogenesis and neurogenesis, a phenomenon recently described by our group. An increase in nerve density is initially observed at the preneoplastic levels and continues in PCa (9). The most powerful recognized relationship between PCa and nerves is PNI. In this article we present data that demonstrates that PNI is the ultimate and most successful neuro-epithelial interaction.
PNI has been described for over 100 years, yet this microscopic finding has been poorly understood, not only biologically, but also as to its predictive potential. The presence of PNI is such a common finding at the microscopic level in neurotropic cancers such as PCa and its sole identification fails to accurately predict clinical behavior. Yet, a closer look at the recently described biology helps us reinterpret the biologic and predictive value of PNI in PCa. Our group has demonstrated conclusively that PNI is involved in the biology of PCa. Early, preneoplastic, interactions between nerves and prostate epithelium have a role in carcinogenesis and morphologically culminate as PNI. As shown by our group PNI has also important roles in other malignancies such as pancreatic (15)and colon cancers. (16, 17) We have not only demonstrated that PNI has a role in cancer biology, but also that PNI has predictive potential.
PNI mimics the interaction between epithelium and nerves. But only when cancer cells successfully utilize the survival mechanisms found in the perineural space, we can truly find biological significance and prediction. Successful PCa cell-nerve interaction is best identified in the amount of cancer cells present in the perineural space, and best measured by the PNI diameter. In this article we explored the influence of PNI diameter on biomarkers expressed by cancer cells located away from the nerves.
In the companion paper we showed that increased nerve density (PGP 9.5 expression) correlated with increased PCa cell proliferation and with proteins implicated in survival pathways, hormone response elements, co-regulators, and repressors. It seems that increased PNI diameter has similar effects on the same pathways. However, they differ in that only PNI diameter, and not nerve density (rho=−0.020; p=0.7260), is very strongly correlated with tumor volume (rho=0.579; p=0.0000). This very strong correlation suggests that axonogenesis is an initial factor, that predisposes and ultimately leads to PNI. Successful interactions between cancer cells and nerves result in greater PNI diameter and eventually induce tumor growth. This successful interaction between nerves and cancer is clearly demonstrated by the predictive value of the PNI diameter, which is an independent predictor of biochemical recurrence 12 and prostate cancer specific death 18. Therefore the ultimate interaction between nerves and cancer cells is PNI, best quantified by the PNI diameter.
In summary, the biology of PNI is clearly involved in the progression of PCa and defines the lethal phenotype.
Footnotes
Disclosure/Conflict of Interest: The authors report no conflict of interest.
The results of this study have been presented in a poster format at the U.S. and Canadian Academy of Pathology meeting, San Antonio, TX, Feb 26 – March 04, 2011.
Funding disclosure:
Funding for this project has been provided by National Cancer Institute through the Tumor Microenvironment Network grant: U54CA126568, project 2 and a Prostate Cancer Foundation Creativity Award.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Villers A, McNeal JE, Redwine EA, Freiha FS, Stamey TA. The role of perineural space invasion in the local spread of prostatic adenocarcinoma. J Urol. 1989;142:763–768. doi: 10.1016/s0022-5347(17)38881-x. [DOI] [PubMed] [Google Scholar]
- 2.Ayala GE, Wheeler TM, Shine HD, Schmelz M, Frolov A, Chakraborty S, Rowley D. In vitro dorsal root ganglia and human prostate cell line interaction: redefining perineural invasion in prostate cancer. Prostate. 2001;49:213–223. doi: 10.1002/pros.1137. [DOI] [PubMed] [Google Scholar]
- 3.Powell MS, Li R, Dai H, Sayeeduddin M, Wheeler TM, Ayala GE. Neuroanatomy of the normal prostate. Prostate. 2005;65:52–57. doi: 10.1002/pros.20245. [DOI] [PubMed] [Google Scholar]
- 4.Ayala GE, Dai H, Ittmann M, Li R, Powell M, Frolov A, Wheeler TM, Thompson TC, Rowley D. Growth and survival mechanisms associated with perineural invasion in prostate cancer. Cancer Res. 2004;64:6082–6090. doi: 10.1158/0008-5472.CAN-04-0838. [DOI] [PubMed] [Google Scholar]
- 5.Cornell RJ, Rowley D, Wheeler T, Ali N, Ayala G. Neuroepithelial interactions in prostate cancer are enhanced in the presence of prostatic stroma. Urology. 2003;61:870–875. doi: 10.1016/s0090-4295(02)02426-3. [DOI] [PubMed] [Google Scholar]
- 6.Ayala GE, Dai H, Tahir SA, Li R, Timme T, Ittmann M, Frolov A, Wheeler TM, Rowley D, Thompson TC. Stromal antiapoptotic paracrine loop in perineural invasion of prostatic carcinoma. Cancer Res. 2006;66:5159–5164. doi: 10.1158/0008-5472.CAN-05-1847. [DOI] [PubMed] [Google Scholar]
- 7.Dai H, Li R, Wheeler T, Diaz de Vivar A, Frolov A, Tahir S, Agoulnik I, Thompson T, Rowley D, Ayala G. Pim-2 upregulation: biological implications associated with disease progression and perinueral invasion in prostate cancer. Prostate. 2005;65:276–286. doi: 10.1002/pros.20294. [DOI] [PubMed] [Google Scholar]
- 8.Ayala GE, Dai H, Li R, Ittmann M, Thompson TC, Rowley D, Wheeler TM. Bystin in perineural invasion of prostate cancer. Prostate. 2006;66:266–272. doi: 10.1002/pros.20323. [DOI] [PubMed] [Google Scholar]
- 9.Ayala GE, Dai H, Powell M, Li R, Ding Y, Wheeler TM, Shine D, Kadmon D, Thompson T, Miles BJ, Ittmann MM, Rowley D. Cancer-related axonogenesis and neurogenesis in prostate cancer. Clin Cancer Res. 2008;14:7593–7603. doi: 10.1158/1078-0432.CCR-08-1164. [DOI] [PubMed] [Google Scholar]
- 10.Cannon GM, Jr, Pound CR, Landsittel DP, Bastacky SI, Dhir R, Becich MJ, Nelson JB. Perineural invasion in prostate cancer biopsies is not associated with higher rates of positive surgical margins. Prostate. 2004 doi: 10.1002/pros.20197. [DOI] [PubMed] [Google Scholar]
- 11.Ng JC, Koch MO, Daggy JK, Cheng L. Perineural invasion in radical prostatectomy specimens: lack of prognostic significance. J Urol. 2004;172:2249–2251. doi: 10.1097/01.ju.0000143973.22897.f8. [DOI] [PubMed] [Google Scholar]
- 12.Maru N, Ohori M, Kattan MW, Scardino PT, Wheeler TM. Prognostic significance of the diameter of perineural invasion in radical prostatectomy specimens. Hum Pathol. 2001;32:828–833. doi: 10.1053/hupa.2001.26456. [DOI] [PubMed] [Google Scholar]
- 13.Li R, Dai H, Wheeler TM, Sayeeduddin M, Scardino PT, Frolov A, Ayala GE. Prognostic value of Akt-1 in human prostate cancer: a computerized quantitative assessment with quantum dot technology. Clin Cancer Res. 2009;15:3568–3573. doi: 10.1158/1078-0432.CCR-08-0826. [DOI] [PubMed] [Google Scholar]
- 14.Li R, Wheeler T, Dai H, Frolov A, Thompson T, Ayala G. High level of androgen receptor is associated with aggressive clinicopathologic features and decreased biochemical recurrence-free survival in prostate: cancer patients treated with radical prostatectomy. Am J Surg Pathol. 2004;28:928–934. doi: 10.1097/00000478-200407000-00013. [DOI] [PubMed] [Google Scholar]
- 15.Dai H, Li R, Wheeler T, Ozen M, Ittmann M, Anderson M, Wang Y, Rowley D, Younes M, Ayala GE. Enhanced survival in perineural invasion of pancreatic cancer: an in vitro approach. Hum Pathol. 2007;38:299–307. doi: 10.1016/j.humpath.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 16.Liebig C, Ayala G, Wilks J, Verstovsek G, Liu H, Agarwal N, Berger DH, Albo D. Perineural invasion is an independent predictor of outcome in colorectal cancer. J Clin Oncol. 2009;27:5131–5137. doi: 10.1200/JCO.2009.22.4949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liebig C, Ayala G, Wilks JA, Berger DH, Albo D. Perineural invasion in cancer: a review of the literature. Cancer. 2009;115:3379–3391. doi: 10.1002/cncr.24396. [DOI] [PubMed] [Google Scholar]