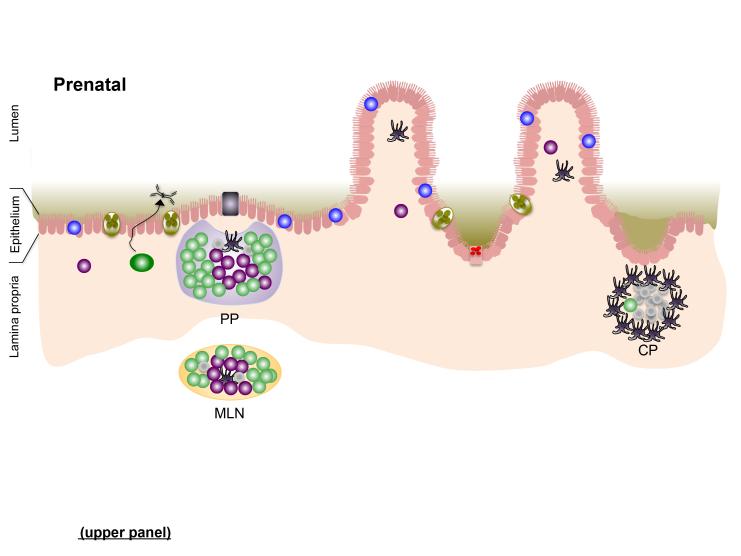
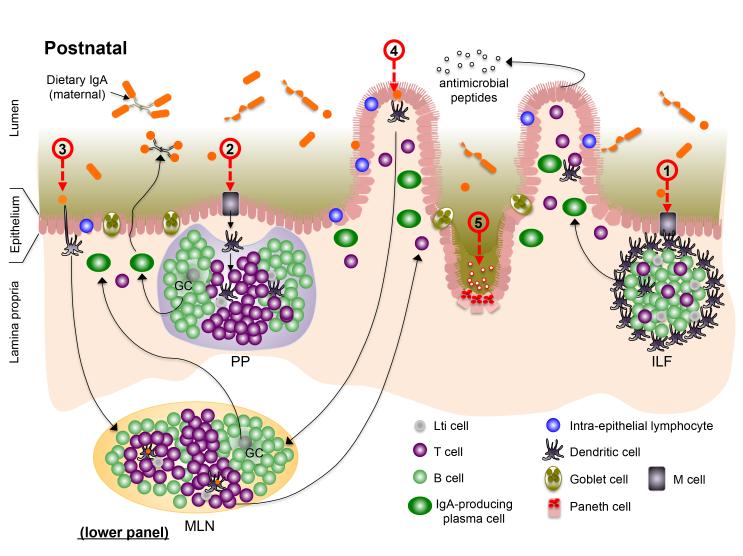
Figure 1. The gut-associated lymphoid tissues (GALT); establishing perinatal host-microbiota mutualism in the intestine.
In utero (prenatal; upper panel), secondary lymphoid tissues [Peyer’s patches (PP) and mesenteric lymph nodes (MLN)], as well as cryptopatches (CP), develop through spatiotemporal recruitment of lymphoid tissue inducer (LTi) cells to sites in the developing intestine and supporting neurovascular structures. The intestinal epithelium is populated by intra-epithelial lymphocytes (IELs) before birth. Bacteria that colonize the neonatal intestine immediately following birth initiate mutiple events that impact development or functional maturation of the mucosa and GALT. Microbe-assoaciated molecular patterns (MAMPs) sensed by patter recognition receptors (PRRs) on intestinal epithelial cells (IECs) and dendritic cells (DCs) adjacent to to cryptopatched (CP) stimultate recruitment of B cells and subsequent maturation of isolated lymphoid follicles (ILFs) (1), which can produce IgA plasma cells via T-dependent and –independent interactions. Increased transport of microbes and their products across the epithelium enter the PP (and ILFs) via M cells (2) before being endocytosed by dendritic cells (DCs) in the subepithelial dome. Antigen-loaded DCs in the PP interact with local lymphocyte subsets to induce T cell differentiation and T-dependent B cell maturation to induce development of IgA-producing plasma cells that home to the lamina propria where they release dimeric IgA for transport into the intestinal lumen. DC-mediated luminal sampling (3) or transcytosis of bacteria across the epithelium (4) results in antigen loading of lamina propria DCs, which migrate via the afferent lymphatics to a draining MLN where they induce differentiation of effector T cells that traffic to the lamina propria. Finally, sensing of MAMPs stimulates the proliferation of IECs in crypts, resulting in their increased depth and, in the small intestine, increased density of Paneth cells and their arming for release of AMPs.