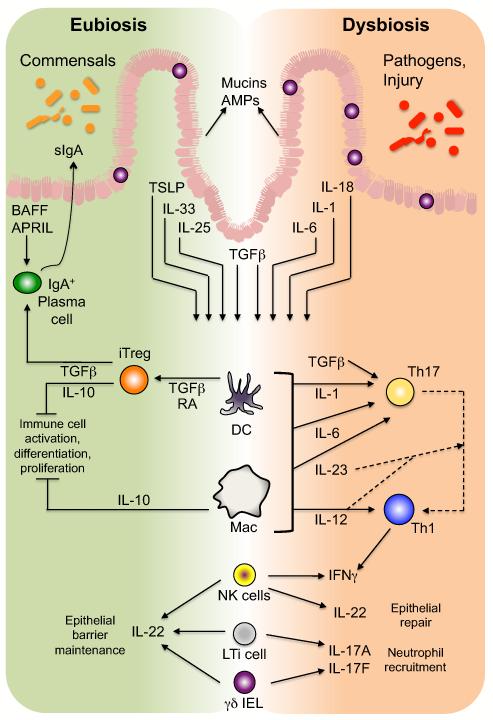
Figure 3. The epithelial-innate-adaptive continuum in the immune response to microbial antigens.
In response to the microbiota, IECs secrete mucins and AMPs that limit microbial interaction with epithelial cells. Also, under homeostatic, eubiotic conditions, epithelial stimulation by microbiota-derived antigens results in secretion of several cytokines (including TSLP, IL-33, IL-25, and TGFβ) that promote development of tolerogenic macrophages (MΦ) and DCs, which induce development of Treg cells via a TGFβ- and RA-dependent process. Intestinal Treg cells, through multiple mechanisms including secretion of TGFβ and IL-10, the latter of which is also produced by a subset of lamina propria macrophages, maintain an anti-inflammatory tone in the intestines by inhibiting or dampening potential effector responses. In addition, Treg cell-derived TGFβ promotes B cell antibody class-switching to IgA, which, coupled with the T-independent induction of IgA in isolated lymphoid follicles via epithelial-derived BAFF and APRIL, ensures an abundant supply of sIgA in the lumen, further limiting microbial interaction with the epithelium. Conversely, in the face of pathogen invasion or dysbiosis, intestinal DCs and macrophages are the targets of multiple IEC-derived pro-inflammatory cytokines and direct interactions with bacteria and/or their MAMPs that induce the development of effector CD4+ T cells, predominantly Th1 and Th17 cells, the latter of which can transition to the former. ILCs in the intestines, including NK-like cells and LTi cells, as well as γδ IELs, respond to cytokine signal derived from IECs and/or myeloid cells (DCs and macrophages) to upregulate cytokines similar to those of effector T cells: IFNγ, IL-17A, and IL-17F, as well as the epithelial barrier protectant, IL-22. Thus, innate cells of the intestinal immune system mediate the equilibrium between anti-inflammatory and pro-inflammatory signals induced by the microbiota under conditions of eubiosis versus dysbiosis, respectively.