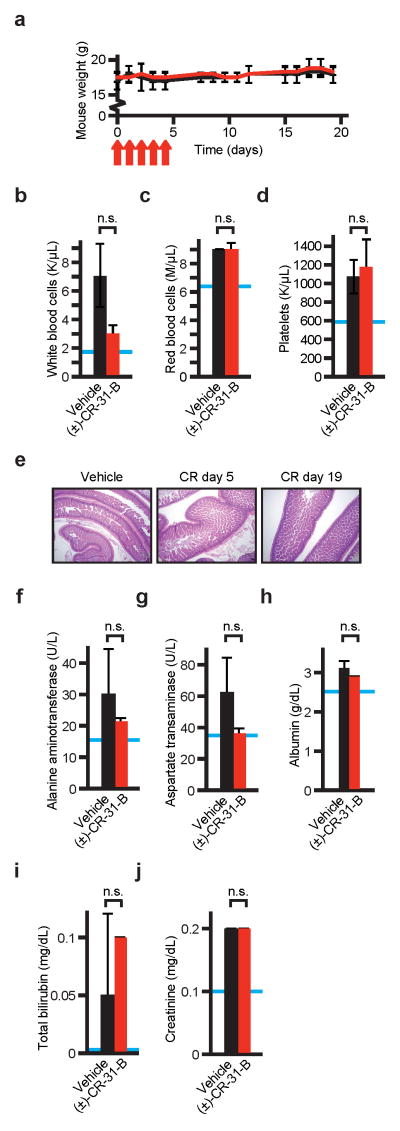
Extended Data Figure 3. a–j) Toxicity studies with (±)-CR-31-B.
Mean and standard deviation are shown, n = 2 biological replicates. a) Animal weights during and after CR treatment (intraperitoneal injection, 0.2 mg/kg on days indicated by red arrows), red = CR, black = vehicle; b–d) Counts of white blood cells (b), red cells (c), and platelets (d) 14 days after cessation of CR treatment, blue lines indicate the species and strain specific reference range, n.s. indicates not significant, n = 2 biological replicates; e) Representative histology of gastrointestinal tract (small intestine) on the indicated days during (n = 4) and after (n = 2) (±)-CR-31-B treatment; f–j) Serum levels of alanine aminotransferase (ALT) (f), aspartate transaminase (AST) (g), albumin (h), total bilirubin (i), and creatinine (j) two weeks after cessation of treatment with CR or vehicle, blue lines indicate the species and strain specific reference range, n.s. indicates not significant.