Table 1.
Chemoselective Aziridination and C–H Amination of Homoallenic Carbamates Catalyzed by Silver Catalysts
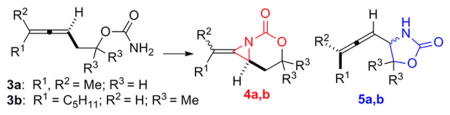
| ||||||||
|---|---|---|---|---|---|---|---|---|
| entrya | catalystb,c |
|
4a | 5a | entrye |
|
4b | 5b |
| 1 | Rh2(esp)2 |
|
35% | 17% | 9 |
|
5% | 80% |
| 2 | AgOTf/phen |
|
79% | --- | 10 |
|
80% | 14% |
| 3 | AgOTf/bipy |
|
60% | --- | 11 |
|
68% | 11% |
| 4 | AgOTf/bathophen |
|
57% | --- | 12 |
|
84% | 12% |
| 5 | AgOTf/p-MeObipy |
|
72% | --- | 13 |
|
73% | 11% |
| 6 | AgOTf/dafone |
|
32% | --- | 14 |
|
59% | 22% |
| 7 | AgOTf/p-Ph-bipy |
|
66% | --- | 15 |
|
62% | 20% |
| 8 | AgOTf/terpy |
|
27% | 35% | 16 |
|
9% | 61% |
Substrate 3a.
5 mol % Rh2(esp)2, 2 equiv PhlO.
Ag: 20 mol % AgOTf, 25 mol % ligand, 2 equiv PhIO, 4 Å MS, CH2Cl2.
A: aziridination. I: insertion.
Substrate 3b.
