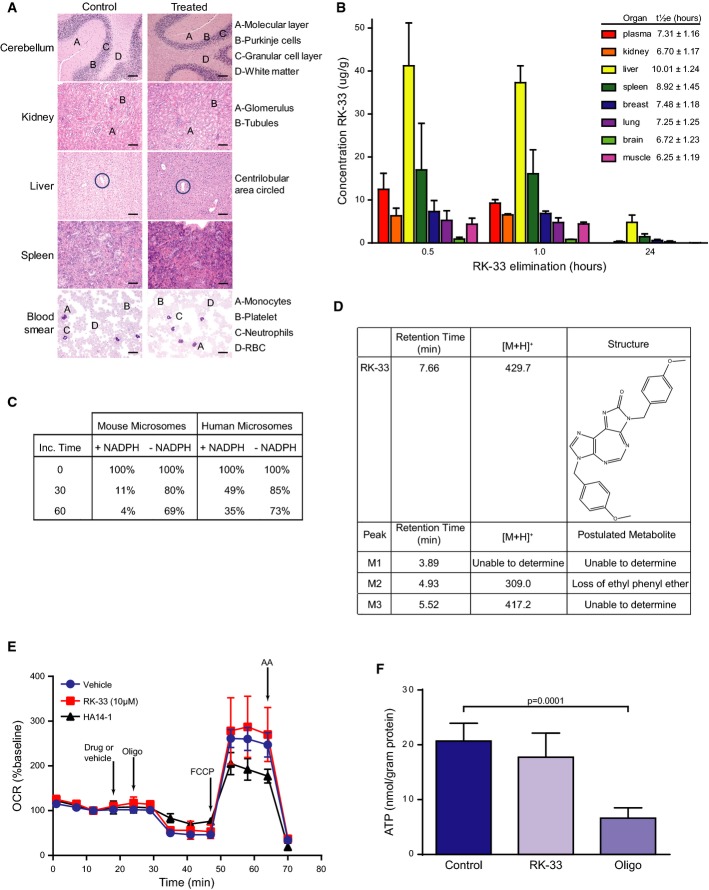
Following injection of 20 mg/kg of RK-33, twice a week for 7 weeks, extensive histopathological examination was carried out following necropsy. Identical patterns were observed both in the control and in the treated mice (n = 2). Samples were stained with H&E. Scale bar is 50 μm.
Pharmacokinetics of RK-33 in SCID mice at various time intervals. Results are mean ± SD from 5 mice. LC-MS/MS method was used to determine concentration of RK-33 in mouse plasma and tissue.
Liquid chromatography–mass spectrometry (LC-MS/MS) analysis was performed to determine different metabolites of RK-33.
RK-33 and metabolites characterized by LC-MS/MS in human liver microsomes using the scan mode function of the LC-MS/MS.
HAPI cells were treated with RK-33 (10 μM), HA14-1 (25 μM), or DMSO vehicle, followed by oligomycin (oligo, 0.5 μg/ml), FCCP (3 μM), and antimycin A (AA, 1 μM) while oxygen consumption rate (OCR) was measured. Pyruvate (10 mM) was added in combination with FCCP to ensure that substrate supply was not rate-limiting for maximal OCR. Data are mean ± SD from 2 to 3 wells and representative of independent experiments performed with two different HAPI passages. OCR is baseline-normalized to the point prior to drug or vehicle addition.
HAPI microglial cells were incubated for 1 h in glucose-free XF24 assay medium that was supplemented with 2-deoxyglucose (50 mM) and pyruvate (10 mM). RK-33 (10 μM), oligomycin (0.5 μg/ml), or vehicle control was additionally present as indicated. Results are mean ± SD from 12 replicates pooled from experiments using two consecutive passages. Significance was assessed by two-sided, unpaired t-test.