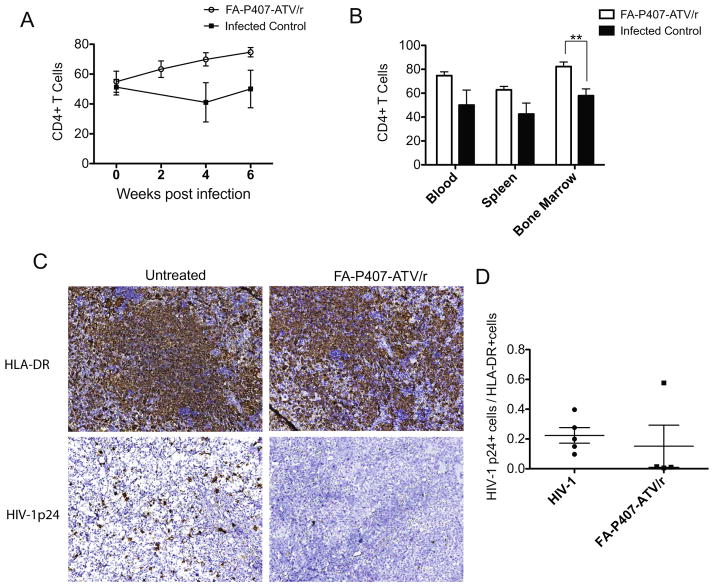
Figure 2. Pharmacodynamics of FA-P407-ATV/r treatment in CD34+ hematopoietic stem cell transplanted NSG mice.
The percent of CD4+ T cells among CD3+ T cells and the CD4+ to CD8+ cell ratios were determined using fluorescence-activated cell sorting between treated (open box) and untreated infected control (closed box) groups. (A) CD4+ T cells in blood during the study and (B) CD4+ T cells in blood, spleen and bone marrow at the end of the study. Data are expressed as mean ± SEM. ** Statistically different at P<0.01 using unpaired Student’s t-test (n=5). Spleen immunohistochemistry was performed for HIV-1p24 antigens following FA-P407-ATV/r treatment. Humanized NSG mice were infected with HIV-1ADA for 10 weeks and then treated with 100 mg/kg FA-P407-ATV/r at 0, 2 and 4 weeks. (C) Spleens were collected from treated and untreated groups and immunostained with antibodies for either HLA-DR or HIV-1p24, then visualized with DAB staining. (D) Antiretroviral efficacy was determined by counting the number of HIV-1p24+ cells and HLA-DR+ cells from five random microscopic fields per mouse and then calculating the HIV-1p24+ to HLA-DR+ cell ratios. Data are expressed, as mean ± SEM (n≥4).