Abstract
3,4-Methylenedioxymethamphetamine (MDMA) is an illicit phenethylamine ingested for entactogenic and euphoric effects. Although blood is more commonly submitted for forensic analysis, previous human MDMA pharmacokinetics research focused on plasma data; no direct blood-plasma comparisons were drawn. Blood and plasma specimens from 50 healthy adult volunteers (33 males, 17 females, 36 African-American) who ingested recreational 1.0 mg/kg and 1.6 mg/kg MDMA doses were quantified for MDMA and metabolites 4-hydroxy-3-methoxymethamphetamine (HMMA), 3,4-methylenedioxyamphetamine (MDA), and 4-hydroxy-3-methoxyamphetamine (HMA) by two-dimensional gas chromatography-mass spectrometry (2D–GCMS). Specimens were collected up to 3 h post-dose and evaluated for maximum concentration (Cmax), first detection time (tfirst), time of Cmax (tmax), and 3-h area under the curve (AUC0–3h); as well as blood metabolite ratios and blood/plasma ratios. Median blood MDMA and MDA Cmax were significantly greater (p<0.0005) than in plasma, but HMMA was significantly less (p<0.0005). HMA was detected in few blood specimens, at low concentrations. Nonlinear pharmacokinetics were not observed for MDMA or MDA in this absorptive phase; but HMMA Cmax and AUC0–3h were similar for both doses despite the 1.6-fold dose difference. Blood MDA/MDMA and MDA/HMMA significantly increased (p<0.0001) over the 3-h time course, and HMMA/MDMA significantly decreased (p<0.0001). Blood MDMA Cmax was significantly greater in females (p=0.010) after the low dose only. Low-dose HMMA AUC0–3h was significantly decreased in females’ blood and plasma (p=0.027), and in African-Americans’ plasma (p=0.035). These data provide valuable insight into MDMA blood-plasma relationships for forensic interpretation, and evidence of sex- and race-based differential metabolism and risk profiles.
Keywords: MDMA, ecstasy, pharmacokinetics, metabolites, blood, plasma
INTRODUCTION
3,4-Methylenedioxymethamphetamine (MDMA, ecstasy) is an illicit amphetamine derivative ingested orally for its entactogenic, stimulant, and hallucinogenic properties [1–5]. In 2010, 0.9 million people took MDMA for the first time in the United States [6]; worldwide, 10–28 million people ages 15–64 have taken the drug. MDMA was found in 0.09% of nighttime drivers in the 2007 US National Roadside Survey [5].
There are two primary Phase I MDMA metabolic pathways. The first is O-demethylenation (mainly mediated by CYP2D6) to 3,4-dihydroxymethamphetamine (HHMA), followed by O-methylation to 4-hydroxy-3-methoxymethamphetamine (HMMA). The second is N-demethylation (CYP1A2) to 3,4-methylenedioxyamphetamine (MDA), followed by O-demethylenation to intermediate 3,4-dihydroxyamphetamine (HHA) and O-methylation to 4-hydroxy-3-methoxyamphetamine (HMA) [7–8].
Administered at recreational doses in human experimental laboratory studies, MDMA increases heart rate (HR) and blood pressure (BP), subjective energy level, closeness to others, and “high,” and causes perceptual changes [9–10]. Subjective effects peak 1–2 h post-administration [9,11]. As a stimulant, some driving performance aspects may improve, but others (e.g., speed adaptation ability) may be impaired [12]. Although blood is a forensically relevant matrix for MDMA and metabolites evaluation, previous clinical and preclinical studies primarily focused on plasma [10,13–14,7,15,11].
We developed a sensitive two-dimensional gas chromatography-mass spectrometry (2D-GCMS) method with cryotrapping for simultaneously quantifying MDMA, HMMA, MDA, HMA, and 3,4-methylenedioxyethylamphetamine (MDEA) in whole blood. We employed this 2D–GCMS method for studying MDMA and metabolite whole blood pharmacokinetics, and correlated MDMA and metabolites blood and plasma concentrations for the first time after controlled MDMA administration. These data will be useful for forensic toxicologists interpreting MDMA blood test results.
MATERIALS AND METHODS
Reagents
MDMA-d0, MDA-d0, MDEA-d0, MDMA-d5, MDA-d5, and MDEA-d6 were purchased from Cerilliant Corp. (Round Rock, TX), racemic HMA-d0 and HMMA-d0 from Lipomed, Inc. (Cambridge, MA), and p-hydroxymethamphetamine (pholedrine) from Sigma (St. Louis, MO). Isopropanol, sodium acetate and ethyl acetate were acquired from Sigma; GC-grade n-heptane, dibasic potassium phosphate, acetic acid, and HPLC-grade methanol were from Fisher Scientific (Fair Lawn, NJ). Hydrochloric acid, sodium hydroxide, ammonium hydroxide, and monobasic potassium phosphate were obtained from JT Baker (Phillipsburg, NJ), triethylamine (>99.5% purity) from Thermo Scientific (Rockford, IL), and heptafluorobutyric acid anhydride (HFAA) from Regis Technologies, Inc. (Morton Grove, IL). Styre Screen™ 6 mL 50 mg DBX solid-phase extraction (SPE) columns were purchased from United Chemical Technologies (Bristol, PA). Blank human plasma and blood were obtained from the National Institutes of Health blood bank.
Participants
Fifty healthy adult participants provided written informed consent for this National Institute on Drug Abuse (NIDA) Intramural Research Program (IRP) Institutional Review Board-approved research (Table 1). Participants underwent comprehensive medical and psychological examinations, including medical and drug histories, as previously described [16]. Eligibility requirements included intake of ≥5 lifetime MDMA tablets (self-report, ≥1 tablet within the past month), ages 18–40 years, and medically acceptable birth control or abstinence (females) throughout the study. Serum pregnancy tests were administered at screening, and urine pregnancy tests preceded each dose administered to females. Exclusion criteria included nursing or pregnant; current medical condition or history of neurologic illness; axis I psychiatric diagnosis other than nicotine, cannabis, or MDMA abuse or dependence; ingestion of a CYP2D6 or CYP3A4 inhibitor or CYP3A4 inducer in the 30 days prior to MDMA administration; abnormal cardiovascular parameters; and serum transaminase greater than three times normal.
Table 1.
Participant demographic data and self-reported current MDMA administration.
| Participant | Race & Ethnicity |
Sex | Age | BMI | Self-reported MDMA Consumption in past 14 daysa |
Self-reported average MDMA tablets per month in past 3 monthsa |
Sessions Completed |
|---|---|---|---|---|---|---|---|
| 1 | U & H | M | 19.7 | 22.4 | 1 | 2 | 3 |
| 2 | B | M | 22.3 | 21.9 | 3 | 1 | 3 |
| 3 | B | M | 19.5 | 26.5 | 2 | 12 | 3 |
| 4 | W | F | 19.4 | 18.6 | 2 | 36 | 3 |
| 5 | B | M | 24.6 | 23.1 | 1 | 3 | 3 |
| 6 | B | F | 26.1 | 34.2 | 5 | 4 | 3 |
| 7 | W | M | 20.5 | 28.2 | 1 | 4 | 3 |
| 8 | W | M | 18.6 | 21.0 | 0 | 2 | 3 |
| 9 | B | M | 20.0 | 27.3 | 8 | 12 | 3 |
| 10 | B | M | 21.2 | 24.8 | 0 | 4 | 3 |
| 11 | B | M | 25.0 | 19.4 | 2 | 2 | 3b |
| 12 | B | F | 20.1 | 28.2 | 4 | 12 | 3 |
| 13 | B | M | 18.3 | 24.4 | 1 | 2 | 3 |
| 14 | B | F | 20.0 | 24.1 | 1c | 4 | 2 |
| 15 | B | F | 22.2 | 28.2 | 10 | 16 | 3 |
| 16 | B | F | 27.6 | 23.3 | 6 | 8 | 3 |
| 17 | B | F | 23.2 | 19.1 | 0 | 32 | 3 |
| 18 | B | F | 21.8 | 18.0 | 1 | 8 | 3 |
| 19 | B | M | 23.8 | 29.5 | 1 | 28 | 3 |
| 20 | B | F | 20.0 | 26.4 | 2 | 2 | 1 |
| 21 | W | F | 20.7 | 18.8 | 1 | 2 | 1 |
| 22 | W | M | 34.9 | 24.4 | 2c | 4 | 3 |
| 23 | B | M | 26.0 | 21.7 | 10 | 96 | 1 |
| 24 | B | M | 28.3 | 28.1 | 4 | 8 | 3 |
| 25 | W | M | 18.5 | 19.7 | 1 | 6 | 3 |
| 26 | W | M | 21.9 | 24.4 | 1 | 2 | 3 |
| 27 | B | M | 24.9 | 31.6 | 1 | 3 | 3 |
| 28 | B & H | F | 23.9 | 35.8 | 6 | 24 | 3 |
| 29 | B | M | 28.6 | 24.2 | 1 | 1 | 3 |
| 30 | B | M | 20.8 | 27.5 | 4 | 12 | 1 |
| 31 | B | F | 21.5 | 31.0 | 4 | 5 | 3 |
| 32 | W | M | 18.9 | 20.7 | 1 | 5 | 3 |
| 33 | W | F | 27.3 | 25.6 | 1 | 0.3 | 2 |
| 34 | B | M | 22.2 | 20.7 | 0 | 2 | 3 |
| 35 | B | M | 25.3 | 19.9 | 1 | 4 | 2 |
| 36 | B | M | 28.5 | 25.7 | 1 | 6 | 3 |
| 37 | B | M | 25.4 | 23.0 | 4 | 36 | 3 |
| 38 | B | M | 22.6 | 22.6 | 0 | 16 | 3 |
| 39 | B | M | 23.7 | 28.2 | 1 | 3 | 1 |
| 40 | B | F | 25.9 | 19.8 | 1 | 0.3 | 3 |
| 41 | B | M | 28.3 | 30.6 | 2 | 16 | 3 |
| 42 | W | F | 24.5 | 22.5 | 0 | 1 | 3 |
| 43 | W | F | 25.9 | 20.5 | 5 | 8 | 3 |
| 44 | B | M | 35.7 | 28.3 | 4 | 16 | 3 |
| 45 | B | M | 27.5 | 30.7 | 4 | 8 | 3 |
| 46 | B | M | 28.7 | 25.1 | 3 | 8 | 3 |
| 47 | B | M | 29.5 | 30.1 | 0 | 0.7 | 1 |
| 48 | B | M | 27.7 | 26.9 | 6 | 20 | 3 |
| 49 | W | F | 23.6 | 25.1 | 1 | 0.3 | 3 |
| 50 | B | M | 28.6 | 25.0 | 5 | 36 | 3 |
| Mean ± SD | 24.1 ± 4.0 | 24.9 ± 4.2 | 2.5 ± 2.4 | 10.9 ± 15.8 | |||
| Median [Range] |
23.8 [18.3–35.7] |
24.6 [18.0–35.8] |
1 [0–10] |
5 [0.3–96] |
|||
Screening data
Participant expectorated high-dose tablet
Participant reported MDMA use as “hallucinogens”
Abbreviations: MDMA, 3,4-methylenedioxymethamphetamine; B, Black or African American; H, Hispanic or Latino; U, Unknown; W, White or Caucasian
Study Procedures
Participants resided on a secure clinical research unit up to 23 days (continuous stay encompassing all dosing sessions, or separate stays separated by ≥1 week). Admission to the residential unit was ≥12 h prior to MDMA dosing. Participants ingested placebo (0 mg/kg), low (1.0 mg/kg), or high (1.6 mg/kg) dose MDMA (Lipomed, Arlesheim, Switzerland) in a randomized, counterbalanced, and double-blind manner. Active drug was administered as the hydrochloride salt; placebo contained lactose. For safety, a maximum 150-mg dose limit was enforced in six participants whose weight exceeded 93.75 kg.
Blood Collection
Blood was collected in sodium heparin (green-top) vacutainers at −0.25, 0.5, 1.0, 1.5, 2.0, 2.5, and 3 h after dosing, and placed on ice immediately after collection. Plasma was collected after centrifuging blood at 2000g, 4°C for 10 min within 2 h after collection. Blood was collected for only 3 h due to a 450 mL maximum total blood collection limit per participant as required by the ethical committee. Extended plasma pharmacokinetics (up to 143 h) from this study were previously published [7]. Blood and plasma were stored frozen (−20°C) until analysis.
Plasma Analysis
MDMA, MDA, HMMA, HMA, and MDEA concentrations were quantified with a validated, modified 2D–GCMS method [17]. Briefly, 1 mL plasma was fortified with internal standard (MDMA-d5, MDA-d5, and pholedrine) and hydrolyzed with 1 mL 0.5 mol/L HCl at 100°C for 40 min. After cooling, 1 mL 0.1 mol/L phosphate buffer (pH 6.0) and 50 µL 10 mol/L sodium hydroxide were added. After centrifugation, supernatant was decanted onto preconditioned polymeric Styre Screen DBX SPE columns. Columns were washed, dried, and extracts eluted with ethyl acetate:isopropanol:ammonium hydroxide (90:6:4, v/v/v). Methanolic HCl (15 µL, 0.12 mol/L) was added prior to evaporation. Residues were reconstituted with 100 µL 0.1 mol/L triethylamine in heptane, and incubated with 10 µL HFAA (derivatizing reagent) at 60°C for 30 min. Phosphate buffer (200 µL, pH 7.4) was added to cooled samples and the aqueous phase discarded. Samples were injected onto an Agilent 6890 GC-5973 mass selective detector instrument equipped with microfluidic Deans switch and flame ionization detector. Instrument parameters were previously reported by Kolbrich et al [17]. Linear ranges were 2.5–400 µg/L (MDMA and HMMA), 2.5–100 µg/L (HMA), and 1–100 µg/L (MDA). Extraction efficiencies were ≥85%, inter- and intra-assay imprecision coefficients of variation (N=20) were ≤6.7%, and analytical bias was between 14.4–7.2% of target.
Blood Analysis
MDMA, MDA, HMMA, HMA, and MDEA were quantified in blood by the plasma method described above with minor modifications. Extraction was performed with 1 mol/L pH 4.5 acetate buffer and reconstitution in 0.2 mol/L triethylamine in heptane.
Method validation was performed to reflect guidelines recently established by the Scientific Working Group for Forensic Toxicology (SWGTOX) [18], and included determining dynamic linear ranges for all compounds, limits of detection (LOD) and quantification (LOQ), recoveries, accuracy and imprecision, endogenous and exogenous interference studies, carryover, stability, and dilution integrity. Parameters were determined with low, medium, and high quality control (QC) samples (Table 2). Detailed validation procedures and results are presented in Online Resource 1. Supplemental Table 1 (within Online Resource 1) provides specific calibration concentrations for each analyte.
Table 2.
Calibration curves, extraction efficiency, and intra- and inter-day imprecision from a 2D–GC/electron impact MS method with cryotrapping for simultaneous MDMA, MDA, HMMA, HMA, and MDEA quantitation in blood.
| MDMA- d5 |
MDMA | MDA- d5 |
MDA | Pholedrine | HMMA | HMA | MDEA- d6 |
MDEA | |
|---|---|---|---|---|---|---|---|---|---|
| Dynamic Range (µg/L) | |||||||||
| IS (MDMA) |
2.5–400 | IS (MDA) |
1.0–100 | IS (HMA, HMMA) |
2.5–400 | 2.5–100 | IS (MDEA) |
2.5–400 | |
| QC Concentrations (µg/L) | |||||||||
| Low | 7.5 | 3.0 | 7.5 | 3.0 | 7.5 | ||||
| Medium | 40 | 15 | 40 | 15 | 40 | ||||
| High | 200 | 75 | 200 | 75 | 200 | ||||
|
Calibration Curve Characteristics, Mean (SD) (n = 7) | |||||||||
| Slope | 0.041 (0.001) |
0.225 (0.012) |
0.020 (0.002) |
0.047 (0.008) |
0.045 (0.001) |
||||
| Intercept | −0.011 (0.003) |
0.115 (0.037) |
0.001 (0.005) |
0.026 (0.016) |
−0.014 (0.002) |
||||
| r^2 | 0.996 (0.002) |
0.997 (0.003) |
0.995 (0.002) |
0.990 (0.007) |
0.996 (0.002) |
||||
|
Extraction Efficiency (%) (n = 6) | |||||||||
| Low | 50.0 | 53.1 | 46.4 | 52.7 | 39.5 | 27.8 | 31.9 | 49.7 | 51.2 |
| Medium | 52.0 | 55.1 | 48.9 | 51.5 | 40.9 | 27.7 | 25.2 | 51.7 | 53.9 |
| High | 54.0 | 57.8 | 53.1 | 56.0 | 43.9 | 30.3 | 27.7 | 53.4 | 55.9 |
|
Inaccuracy (%) (N = 21) | |||||||||
| Low | 0.2 | 10.0 | 2.4 | −1.6 | −1.4 | ||||
| Medium | −0.5 | 8.7 | 4.0 | −0.3 | 2.3 | ||||
| High | 5.3 | 2.6 | 10.9 | 0.8 | 5.4 | ||||
|
Intra-Day Imprecision (%) (n = 3) | |||||||||
| Low | 1.9 | 3.5 | 2.5 | 5.9 | 1.7 | ||||
| Medium | 2.0 | 2.3 | 3.2 | 3.5 | 2.2 | ||||
| High | 1.5 | 2.1 | 3.6 | 7.3 | 1.5 | ||||
|
Inter-Day Imprecision (%) (N = 21) | |||||||||
| Low | 2.9 | 4.8 | 5.0 | 6.3 | 2.6 | ||||
| Medium | 2.9 | 3.6 | 5.6 | 5.6 | 2.9 | ||||
| High | 3.6 | 2.6 | 5.1 | 8.6 | 3.5 | ||||
Abbreviations: 2D–GC, 2-dimensional gas chromatography; MS, mass spectrometry; MDMA, 3,4-methylenedioxymethamphetamine; MDA, 3,4-methylenedioxyamphetamine; HMMA, 4-hydroxy-3-methoxymethamphetamine; HMA, 4-hydroxy-3-methoxyamphetamine; MDEA, 3,4-methylenedioxyethylamphetamine
Data Analysis
Statistical analyses were performed with IBM® SPSS® Statistics 19.0.0 for Windows. Data displayed non-normal distribution by visual inspection and Kolmogorov-Smirnov tests; thus, nonparametric analyses were utilized. Observed time of first detection (tfirst), maximum concentration (Cmax), time of maximum concentration (tmax), and 3-h area under the curve (AUC0–3h, calculated by linear trapezoidal method) were compared with the Wilcoxon signed-ranks test. Between-group sex and race comparisons were performed with the Mann-Whitney U test. Because only two participants identified as unknown/Hispanic and/or mixed-race/ethnicity (insufficient for additional categories), these individuals were included in a group with white participants. To avoid ambiguity, cases where no analyte was detected were excluded from tfirst and tmax calculations. Blood/plasma ratios were calculated only when analytes were quantifiable in both matrices. Low and high dose blood/plasma ratios, overall and by time, were compared with the Wilcoxon signed-ranks test. Analyte ratios (MDA/MDMA, HMMA/MDMA, and MDA/HMMA) also were evaluated with Wilcoxon signed-ranks test, and temporal differences were evaluated by independent samples median test. Linear least-squares plasma vs. blood concentration (MDMA, MDA, HMMA) regressions were evaluated in GraphPad Prism 5.02 (GraphPad Software Inc.).
RESULTS
Participants
Fifty volunteers (33 males, 17 females) participated; 41 completed all three sessions. Demographic and amphetamines use history are presented in Table 1.
Blood Method
The optimized blood MDMA and metabolites assay was achieved by modifying the plasma method to optimize the pH for extraction and triethylamine concentration for derivatization. Extraction at pH (6.0) utilized for plasma analyses revealed poor HMA extraction efficiency with non-detectable HMA at 3.0 µg/L; >25.2% HMA extraction efficiency was achieved with pH 4.5 buffer [19]. Validation data are presented in Table 2 and Online Resource 1.
Blood and Plasma Specimens
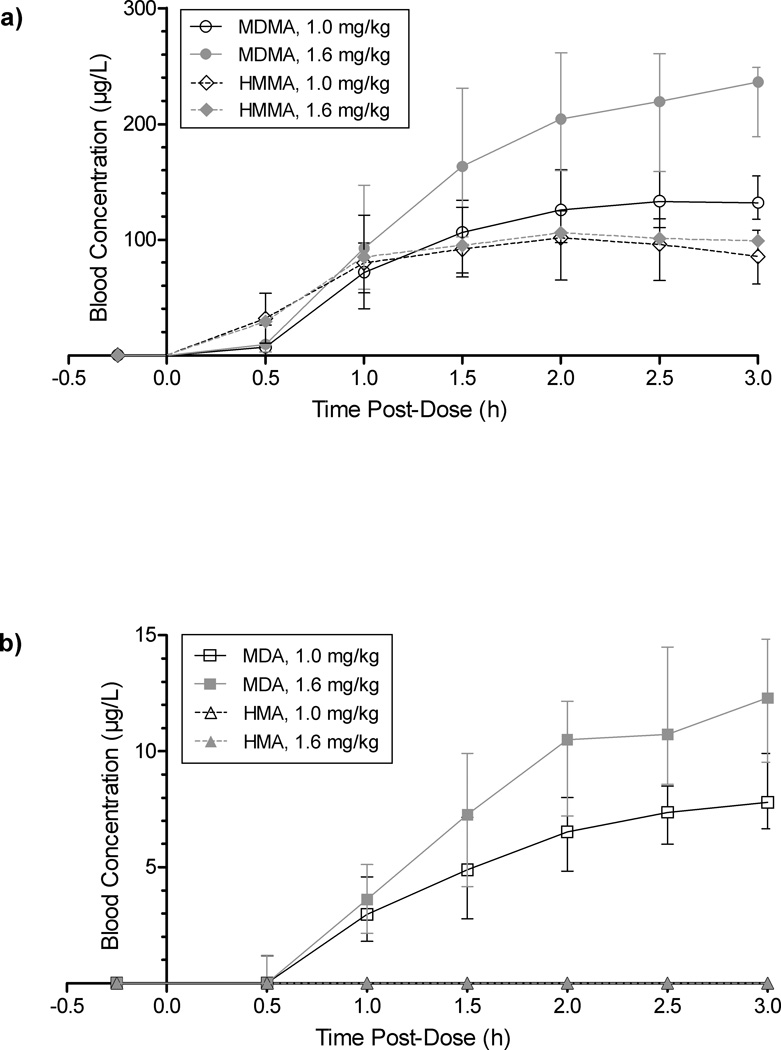
Blood (144 after placebo, 321 after low, and 297 after high dose MDMA) and plasma (145 after placebo, 321 after low, and 300 after high dose MDMA) specimens were analyzed for MDMA, HMMA, MDA, and HMA. There were 757 (144 placebo, 316 low, 297 high) concurrently collected blood and plasma specimens. Specimens were analyzed until there were three consecutive negative specimens, resulting in fewer total placebo analyses. Rigorous plasma pharmacokinetic analysis (17 participants, up to 143 h post-dose) was previously published [7]. Blood was collected up to 3 h post-dose. Fig. 1 depicts blood time courses for MDMA and metabolites. Metabolite HMMA concentrations were similar after the two doses, but MDMA and MDA concentrations were dose-dependent. HMA was only detected in 4 blood specimens.
Fig. 1.
Median (interquartile range) a) 3,4-methylenedioxymethamphetamine (MDMA) and 4-hydroxy-3-methoxymethamphetamine (HMMA); and b) 3,4-methylenedioxyamphetamine (MDA) and 4-Hydroxy-3-methoxyamphetamine (HMA), in blood over 3 h after oral 1.0 and 1.6 mg/kg MDMA administration to adult users
MDMA and MDA
Median blood MDMA and MDA Cmax were significantly greater than in plasma (p<0.0005, Table 3). Overall median MDMA blood and plasma Cmax achieved after the high dose were 1.7 and 1.6 times those after the low dose, reflecting the 1.6-fold difference in MDMA administered. Median MDA 3 h observed Cmax in both blood and plasma also were 1.6-fold higher after the high dose. Median tmax occurred at 2.5 h for MDMA and 3.0 h for MDA, consistent with the demethylation process requiring additional time to peak. Similarly, tfirst was non-significantly earlier (0.5 h) for the parent compound than the metabolite (1.0 h); and MDA after the low, but not high, dose revealed a significantly earlier tfirst (p=0.002) in blood than plasma. Median MDMA AUC0–3h were 1.7 and 1.6-fold higher in blood and plasma after the high than low dose, respectively, consistent with Cmax. Median MDA AUC0–3h high v. low dose ratios were lower (1.5 and 1.4 for blood and plasma, respectively).
Table 3.
Median [range] MDMA, MDA, HMMA and HMA observed Cmax, tmax, tfirst, and AUC0–3h in blood and plasma after 1.0 (low) and 1.6 (high) mg/kg controlled oral MDMA administration.
| Blood | N | Plasma | N | p-value (blood vs. plasma) |
Blood | N | Plasma | N | p-value (blood vs. plasma) |
||
|---|---|---|---|---|---|---|---|---|---|---|---|
| MDMA | MDA | ||||||||||
| Observed Cmax (µg/L) | |||||||||||
| Overall | Low | 144.9 [90.3–358.2] | 46 | 126.3 [66.9–276.1] | 46 | <0.0005 | 8.0 [4.0–18.3] | 46 | 5.5 [2.3–11.3] | 46 | <0.0005 |
| High | 241.6 [58.5–461.9] | 41 | 205.1 [46.3–465.3] | 41 | <0.0005 | 13.0 [3.0–24.1] | 41 | 8.6 [2.0–21.0] | 42 | <0.0005 | |
| p-value (low vs. high) | <0.0005 | <0.0005 | <0.0005 | <0.0005 | |||||||
| Females | Low | 159.0b [111.7–239.3] | 13 | 129.8 [97.0–243.6] | 13 | 8.0 [6.0–11.7] | 13 | 6.1 [3.7–8.4] | 13 | ||
| High | 240.2 [153.6–461.9] | 14 | 194.6 [125.6–465.3] | 15 | 12.5 [4.4–24.1] | 14 | 8.5 [2.6–21.0] | 15 | |||
| Males | Low | 131.9b [90.3–358.2] | 32 | 120.1 [66.9–276.1] | 32 | 8.0 [4.0–18.3] | 32 | 5.3 [2.3–8.5] | 32 | ||
| High | 241.6 [58.5–390.9] | 27 | 205.5 [46.3–361.9] | 27 | 13.0 [3.0–20.4] | 27 | 8.7 [2.0–12.9] | 27 | |||
|
African American |
Low | 144.4 [90.3–358.2] | 33 | 127.3 [66.9–276.1] | 33 | 8.0 [5.3–18.3] | 33 | 5.3 [2.6–8.4] | 33 | ||
| High | 244.0 [117.0–461.9] | 28 | 213.4 [95.6–465.3] | 28 | 12.7 [4.4–24.1] | 28 | 8.8 [3.7–21.0] | 28 | |||
|
White, Hispanic, or Mixeda |
Low | 137.7 [91.1–176.4] | 12 | 113.3 [74.5–164.4] | 12 | 7.9 [4.0–11.7] | 12 | 5.9 [2.3–8.5] | 12 | ||
| High | 241.0 [58.5–345.3] | 13 | 199.6 [46.3–296.4] | 14 | 13.0 [3.0–19.2] | 13 | 7.8 [2.0–12.6] | 14 | |||
| Observed tmax (h) | |||||||||||
| Low | 2.5 [1.5–3.0] | 46 | 2.5 [1.5–3.0] | 46 | ns | 3.0 [1.5–3.0] | 45 | 3.0 [2.0–3.0] | 46 | ns | |
| High | 2.5 [1.0–3.0] | 41 | 2.5 [1.0–3.0] | 42 | ns | 3.0 [1.5–3.0] | 41 | 3.0 [2.0–3.0] | 42 | ns | |
| p-value (low vs. high) | ns | ns | ns | ns | |||||||
| Observed tfirst (h) | |||||||||||
| Low | 0.5[−0.25–1.5] | 47 | 0.5[−0.25–1.5] | 46 | ns | 1.0[−0.25–2.0] | 47 | 1.0[−0.25–2.5] | 46 | 0.002 | |
| High | 0.5[−0.25–2.0] | 41 | 0.5[−0.25–2.5] | 42 | ns | 1.0 [0.5–3.0] | 41 | 1.0 [0.5–3.0] | 42 | ns | |
| p-value (low vs. high) | ns | ns | ns | ns | |||||||
| AUC0–3h (h•µg/L) | |||||||||||
| Overall | Low | 248.9 [120.0–536.4] | 45 | 220.7 [98.7–480.7] | 45 | <0.0005 | 12.9 [4.1–26.4] | 45 | 8.8 [2.5–15.2] | 45 | <0.0005 |
| High | 419.0 [20.0–831.7] | 40 | 352.7 [16.2–846.3] | 42 | <0.0005 | 19.4 [0.8–43.6] | 40 | 11.9 [0.5–36.6] | 42 | <0.0005 | |
| p-value (low vs. high) | <0.0005 | <0.0005 | <0.0005 | <0.0005 | |||||||
| Females | Low | 279.3 [148.8–469.2] | 14 | 237.3 [119.8–480.7] | 14 | 13.9 [6.7–18.3] | 14 | 9.1 [2.6–15.2] | 14 | ||
| High | 450.8 [187.7–818.2] | 14 | 384.9 [85.0–846.3] | 15 | 18.7 [6.9–43.6] | 14 | 11.8 [0.7–36.6] | 15 | |||
| Males | Low | 235.6 [120.0–536.4] | 32 | 212.0 [98.7–441.3 | 32 | 12.5 [4.1–26.4] | 32 | 8.0 [2.5–13.9] | 32 | ||
| High | 413.7 [20.0–831.7] | 26 | 341.6 [16.2–734.9] | 27 | 19.5 [0.8–39.0] | 26 | 12.4 [0.5–20.4] | 27 | |||
|
Black or African American |
Low | 251.3 [120.0–536.4] | 34 | 223.9 [98.7–480.7] | 34 | 13.4 [6.6–26.4] | 34 | 8.9 [2.6–15.2] | 34 | ||
| High | 419.1 [192.4–831.7] | 27 | 352.7 [180.9–846.3] | 28 | 19.07 [6.9–43.6] | 27 | 12.9 [3.4–36.6] | 28 | |||
|
White, Hispanic, or Mixeda |
Low | 245.9 [126.4–326.5] | 12 | 189.9 [107.7–302.3] | 12 | 12.8 [4.1–19.0] | 12 | 8.7 [2.5–12.4] | 12 | ||
| High | 418.8 [20.0–668.0] | 13 | 352.9 [16.2–581.1] | 14 | 20.0 [0.8–37.4] | 13 | 11.0 [0.5–20.4] | 14 | |||
| HMMA | HMA | ||||||||||
| Observed Cmax (µg/L) | |||||||||||
| Overall | Low | 107.0 [24.9–231.1] | 46 | 143.7 [35.6–304.9] | 46 | <0.0005 | 0.0 [0.0–3.1] | 46 | 0.0 [0.0–3.2] | 46 | 0.070 |
| High | 111.0 [19.8–222.3] | 41 | 147.5 [19.8–299.2] | 42 | <0.0005 | 0.0 [0.0–2.6] | 41 | 0.0 [0.0–3.5] | 42 | 0.012 | |
| p-value (low vs. high) | ns | ns | ns | ns | |||||||
| Females | Low | 98.1 [24.9–231.1] | 13 | 117.7 [35.6–259.5] | 13 | 0.0 [0.0–3.1] | 13 | 0.0 [0.0–2.7] | 13 | ||
| High | 109.8 [19.8–222.3] | 14 | 135.6 [19.8–265.1] | 15 | 0.0 [0.0–2.6] | 14 | 0.0 [0.0–3.2] | 14 | |||
| Males | Low | 110.1 [49.0–211.5] | 32 | 153.5 [67.3–304.9] | 32 | 0.0 [0.0–0.0] | 32 | 0.0 [0.0–3.2] | 32 | ||
| High | 111.0 [53.4–197.9] | 27 | 161.2 [78.8–299.2] | 27 | 0.0 [0.0–2.5] | 27 | 0.0 [0.0–3.5] | 27 | |||
|
Black or African American |
Low | 105.6 [24.9–211.5] | 33 | 125.8 [35.6–304.9] | 33 | 0.0 [0.0–0.0] | 33 | 0.0 [0.0–3.2] | 33 | ||
| High | 113.6 [19.8–197.9] | 28 | 147.5 [19.8–299.2] | 28 | 0.0 [0.0–2.5] | 28 | 0.0 [0.0–3.5] | 28 | |||
|
White, Hispanic, or Mixeda |
Low | 112.2 [33.1–231.1] | 12 | 174.6 [42.0–259.5] | 12 | 0.0 [0.0–3.1] | 12 | 0.0 [0.0–2.9] | 12 | ||
| High | 111.0 [73.9–222.3] | 13 | 152.1 [85.3–265.1] | 14 | 0.0 [0.0–2.6] | 13 | 0.0 [0.0–2.8] | 14 | |||
| Observed tmax (h) | |||||||||||
| Low | 2.0 [1.0–3.0] | 46 | 2.0 [1.0–3.0] | 46 | ns | 3.0 | 1 | 3.0 [2.0–3.0] | 8 | -- | |
| High | 2.0 [1.0–3.0] | 41 | 2.0 [1.0–3.0] | 42 | ns | 3.0 [3.0–3.0] | 2 | 3.0 [2.5–3.0] | 9 | -- | |
| p-value (low vs. high) | ns | ns | -- | -- | |||||||
| Observed tfirst (h) | |||||||||||
| Low | 0.5[−0.25–1.0] | 47 | 0.5[−0.25–1.0] | 46 | ns | 3.0 | 1 | 2.0 [1.0–3.0] | 8 | -- | |
| High | 0.5[−0.25–2.0] | 42 | 0.5[−0.25–2.0] | 42 | ns | 2.5 [2.0–3.0] | 2 | 2.5 [1.5–3.0] | 9 | -- | |
| p-value (low vs. high) | ns | ns | -- | -- | |||||||
| AUC0–3h (h•µg/L) | |||||||||||
| Overall | Low | 217.4 [40.7–459.9] | 45 | 302.0 [59.4–596.0] | 45 | <0.0005 | 0.0 [0.0–0.8] | 45 | 0.0 [0.0–6.5] | 45 | 0.012 |
| High | 249.4 [37.8–500.1] | 40 | 322.0 [37.8–646.8] | 42 | <0.0005 | 0.0 [0.0–1.9] | 40 | 0.0 [0.0–5.1] | 42 | 0.014 | |
| p-value (low vs. high) | ns | ns | ns | ns | |||||||
| Females | Low | 200.9c [40.7–450.5] | 14 | 256.3c [59.4–477.4] | 14 | 0.0 [0.0–0.8] | 14 | 0.0 [0.0–2.0] | 14 | ||
| High | 233.9 [37.8–500.1] | 14 | 270.8 [37.8–588.4] | 15 | 0.0 [0.0–1.9] | 14 | 0.0 [0.0–5.1] | 15 | |||
| Males | Low | 238.6c [95.6–459.9] | 32 | 333.2c [128.6–596.0] | 32 | 0.0 [0.0–0.0] | 32 | 0.0 [0.0–6.5] | 32 | ||
| High | 250.1 [87.8–470.8] | 26 | 349.1 [120.9–646.8] | 27 | 0.0 [0.0–0.6] | 26 | 0.0 [0.0–4.0] | 27 | |||
|
Black or African American |
Low | 203.0 [40.7–459.9] | 34 | 271.9d [59.4–596.0] | 34 | 0.0 [0.0–0.0] | 34 | 0.0 [0.0–6.5] | 34 | ||
| High | 252.3 [37.8–470.8] | 27 | 322.0 [37.8–646.8] | 28 | 0.0 [0.0–0.6] | 27 | 0.0 [0.0–5.1] | 28 | |||
|
White, Hispanic, or Mixeda |
Low | 253.4 [55.4–450.5] | 12 | 375.5d [67.1–511.8] | 12 | 0.0 [0.0–0.8] | 12 | 0.0 [0.0–3.5] | 12 | ||
| High | 246.5 [87.8–500.1] | 13 | 321.5 [82.4–588.4] | 14 | 0.0 [0.0–1.9] | 13 | 0.0 [0.0–2.1] | 14 | |||
Abbreviations: MDMA, 3,4-methylenedioxymethamphetamine; MDA, 3,4-methylenedioxyamphetamine; HMMA, 4-hydroxy-3-methoxymethamphetamine; HMA, 4-hydroxy-3-methoxyamphetamine; Cmax, observed maximum concentration; tmax, time of observed Cmax; tfirst, time of first detection; AUC0–3h, 3 h area under the curve; ns, not significant.
Incorporated into one category due to insufficient number identifying as Hispanic or Mixed
Low blood MDMA Cmax was significantly higher in females vs. males (p=0.010).
Low blood and plasma HMMA AUC0–3h was significantly lower in females vs. males (p=0.027 blood and p=0.027 plasma).
Low plasma AUC0–3h was significantly lower in African-Americans vs. white/Hispanic or mixed race individuals (p=0.035).
Median MDMA blood Cmax was significantly greater in females than males (p=0.010) after the low dose only. Plasma Cmax and AUC0–3h did not differ significantly by sex for MDMA or MDA; nor did blood MDA Cmax or AUC0–3h. No racial differences in MDMA or MDA Cmax or AUC0–3h were observed.
Two participants (16 and 24) had consistent and quantifiable MDMA from baseline throughout the placebo session (Online Resource 2). Self-reported last-consumed MDMA was 2 days (16) and 16–20 h (24) prior to first specimen collection. MDA was detected in both individuals’ plasma, and in blood for participant 16 only. Participant 11 chewed and expectorated the high-dose capsule. No MDMA, MDA, or HMA was detected in his blood or plasma. HMMA was still positive from a previous dose; and concentrations remained within ±16% and ±9% of baseline for blood and plasma, respectively. This session was excluded from tfirst, Cmax, and tmax calculations.
HMMA and HMA
Blood HMMA Cmax was significantly less than in plasma (p<0.0005) at both MDMA doses (Table 3). HMMA concentrations were not linear with dose, achieving median high-dose Cmax and AUC0–3h 1.0 and 1.1 times those of the low dose (Table 3, Fig. 1a). Tmax and tfirst did not differ between doses or matrices. Few specimens were positive for HMA (blood, n=4/618; plasma, n=41/621). HMA only slightly exceeded the 2.5 µg/L LOQ when positive (Cmax 3.1, 2.6 µg/L [low, high blood]; 3.2, 3.5 µg/L [low, high plasma]). The trend (p=0.070) for slightly lower HMA concentrations in blood vs. plasma after the low dose became significant (p=0.012) at the high dose. There were too few positive HMA specimens to statistically compare doses or matrices for tfirst or tmax.
The only significant sex difference observed was with HMMA concentrations after the low MDMA dose, which showed lower AUC0–3h in females than males (p=0.027, blood and plasma). Also for the low dose only, plasma HMMA AUC0–3h was significantly lower (p=0.035) in African-American participants. No blood matrix or high-dose racial differences were observed.
MDEA
The MDMA capsules administered in the study did not contain MDEA, and no MDEA was detected in blood or plasma.
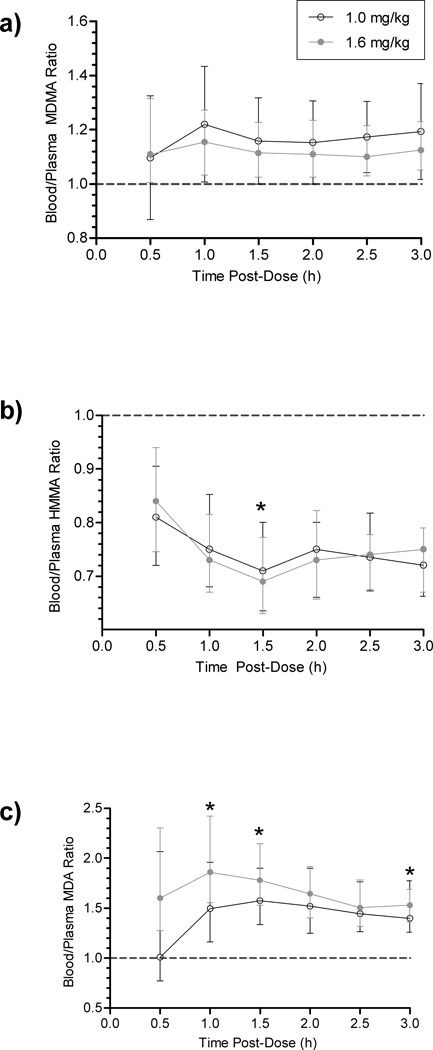
Blood/Plasma Ratios
Median [range] blood/plasma ratios for paired low and high-dose MDMA (N=253 and 236), HMMA (N=267 and 250), and MDA (N=212 and 199) were 1.17 [0.42–1.89] and 1.12 [0.86–2.33]; 0.75 [0.45–1.12] and 0.74 [0.03–1.44]; and 1.49 [0.63–4.83] and 1.63 [0.60–5.43], respectively. At most time points, blood/plasma ratios did not vary significantly by dose (Fig. 2). In this primarily-absorptive phase, only MDA displayed a significantly greater overall blood/plasma ratio after the high than the low dose (p<0.0005). Blood MDMA and MDA concentrations were always greater than plasma (ratio >1); HMMA blood concentrations were always less than those of plasma. Despite substantial inter-subject variability, ratios were relatively consistent over time, particularly MDMA. Linear least-square regression equations for blood-plasma concentration relationships were y=0.9287x-5.280 (MDMA), y=0.5214x+0.7891/y=0.6570x-0.1834 (MDA low/high), and y=1.342x+3.094 (HMMA), with respective R2=0.9512, 0.5889/0.7409, and 0.8964 (Online Resource 3). All correlations were significant (p<0.0001). There was only one paired HMA specimen (high dose, t=3 h, blood/plasma=0.72).
Fig. 2.
Median (interquartile range) blood/plasma 3,4-methylenedioxymethamphetamine (MDMA) (a), 4-hydroxy-3-methoxymethamphetamine (HMMA) (b), and 3,4-methylenedioxyamphetamine (MDA) (c) ratios for 3 h after controlled MDMA administration. Changes over time were significant after the 1.6 mg/kg dose for HMMA and MDA (p=0.013 and p=0.021), but not for MDMA. No changes over time were significant after the 1.0 mg/kg dose. Note: y-axes do not begin at 0. *Denotes p<0.05 (low vs. high)
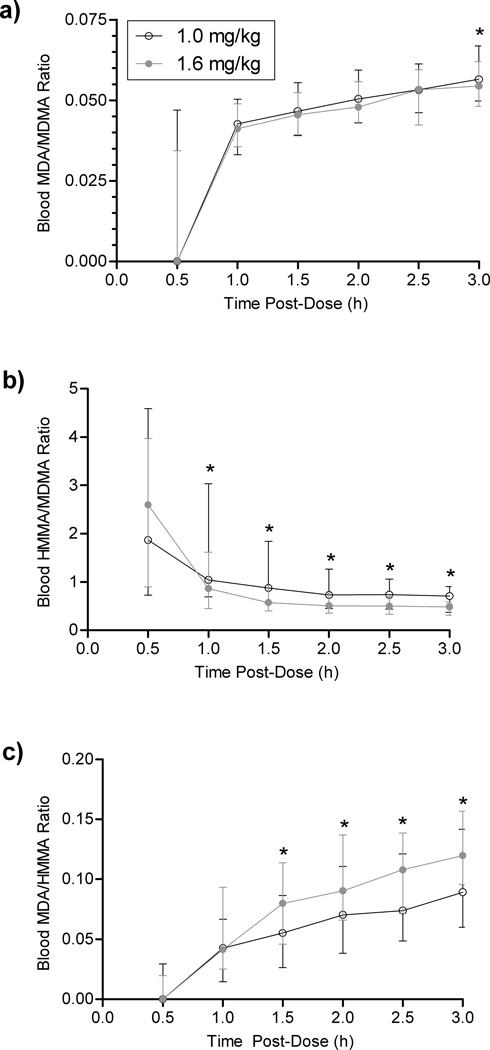
Analyte Ratios
Blood MDA/MDMA (Fig. 3a) and MDA/HMMA (Fig. 3c) ratios significantly increased (p<0.0001) over the 3-h time course; whereas HMMA/MDMA ratios significantly decreased overall (p<0.0001), but were relatively stable after the first h post-dose (Fig. 3b). Low vs. high-dose time-course slopes were not significantly different for MDA/MDMA, MDA/HMMA, or HMMA/MDMA. Beginning at 1.0 h, HMMA/MDMA was significantly greater (p<0.005) after the low than the high dose, and after 1.5 h MDA/HMMA was significantly greater (p<0.005) after the high dose. MDA in blood was almost twenty-fold lower than blood MDMA. Overall MDA/MDMA, MDA/HMMA, and HMMA/MDMA ratios all displayed significant dose differences (p<0.0005), although mean low vs. high dose MDA/MDMA differed by only 8.5% (0.052 vs. 0.047). Thus, despite statistical significance, this difference was not clinically significant. Mean MDA/MDMA low vs. high dose was 26.7% greater (1.46 vs. 1.15), and mean MDA/HMMA low vs. high was 21.3% lower (0.088 vs. 0.111).
Fig. 3.
Median (interquartile range) blood MDA/MDMA (a), HMMA/MDMA (b), and MDA/MDMA (c) ratios over 3 h after controlled MDMA administration. *Denotes significant difference (low vs. high MDMA dose), p<0.05. Changes over time were significant (p<0.0005, p<0.01, and p<0.01 for MDA/MDMA, HMMA/MDMA, and MDA/HMMA, respectively). Abbreviations: MDA, 3,4-methylenedioxyamphetamine; MDMA, 3,4-methylenedioxymethamphetamine; HMMA, 4-hydroxy-3-methoxymethamphetamine
DISCUSSION
We established and validated a sensitive, specific 2D-GCMS method for simultaneously quantifying MDMA, MDA, HMMA, HMA, and MDEA in blood and utilized this method to analyze specimens after controlled MDMA administration. We believe this to be valuable analytically novel information in that it contains, to our knowledge, the first published whole blood method for simultaneous quantification of MDMA and its metabolites HMMA, MDA, and HMA, as well as MDEA [20,14,21–27]. MDMA and MDA are weak bases (pKa approximately 9.9 [28] and 9.67 [29], respectively); as are probably HMA and HMMA, based on their amine groups. Thus, these analytes were more effectively protonated (ionized) at the lower extraction pH, allowing more consistent cation exchange SPE column functionality. Doubling the triethylamine concentration in the reconstitution solvent also improved HMA quantification. Extraction efficiencies from blood were substantially diminished compared to our previously-published plasma results [17]. We attempted to improve recovery by testing QC specimens extracted at pH 6 (n=3), and after protein-precipitation with ice-cold acetonitrile after hydrolysis (n=3); neither condition improved extraction efficiency. We were able to achieve desired sensitivity with acceptable analytical performance despite low analyte recoveries. The recent publication of the SWGTOX guidelines on method validation were followed. These new comprehensive guidelines demonstrate proper validation procedures in a concise and simplified manner. This information combining both analysis and interpretation of blood MDMA following controlled MDMA administration will benefit the field.
We present, for the first time, in vivo human blood vs. plasma relationships for MDMA and metabolites MDA, HMMA, and HMA. We also examined metabolite ratios in blood, and directly compared sex and racial differences in MDMA pharmacokinetics. This study was limited by the 3 h post-dose blood collection window imposed by a total blood collection limit of 450 mL per participant over the entire study. This prevented extended blood pharmacokinetic analysis. In cases where tmax was reported as 3 h, the true tmax could have been later; Cmax were observed 3 h data. Another limitation was long-term storage prior to analysis. Although stored frozen at −20°C in the dark, blood and some plasma specimens were not analyzed until 1–7 years after collection. MDMA and MDA were determined to be stable frozen ≥17 weeks in serum and 5 weeks in blood [30–31], but longer-term MDMA and metabolites stabilities are not fully characterized
To our knowledge, blood-plasma relationships were previously determined in only two in vitro studies [31–32]. MDMA and MDA concentrations were significantly (p<0.0005) higher in blood than plasma (Table 3, Fig. 3). Garrett et al [31] documented similar red blood cell (RBC)-plasma coefficients for MDMA and MDA (1.48 and 1.45, respectively), corroborating our findings. Values are higher than the blood/plasma ratios we determined, possibly because reported results were partition coefficients for separated RBCs vs. plasma rather than whole blood vs. plasma concentrations. Plasma-protein binding was reported to be 34–40% for both compounds. In contrast, Belhadj-Tahar et al [32] reported the (haematocrit-normalized) RBC vs. whole blood partition coefficient for MDA (MDMA not reported) as 30%, the free fraction 27%, and the plasma protein-bound fraction 71%.
We found significant dose-dependent differences in MDA blood/plasma ratios and concentration correlations (Fig. 2, Online Resource 3). Based on previous findings [31], which showed MDA and MDMA plasma-protein binding was not dose-dependent, the relative increase may relate more to RBC affinity than to plasma-protein binding. Taken together, these results suggest that higher concentrations may gradually exceed RBC binding capacity, allowing more free drug to partition into plasma. Correlation coefficients were relatively high for MDMA (0.9512) and HMMA (R2=0.8964) and somewhat lower for MDA (>0.58). This suggests greater inter-subject variability in MDA blood-plasma partitioning than for MDMA and HMMA, possibly contributing to these findings.
To our knowledge, no HMMA or HMA partition coefficients were reported to date, but our results (blood-plasma ratio <1 and significantly lower blood Cmax) indicate HMMA partitions more readily into plasma. The significant HMMA blood/plasma ratio dose difference observed at 1.5 h (Fig. 2) represented a minor fluctuation likely caused by inter-subject variability, and appears to lack clinical significance. HMA is a relatively minor metabolite, with concentrations never exceeding 3.5 µg/L during the 3-h time course, even after the high dose. The single paired-positive HMA result (0.72 blood/plasma ratio) agreed with the slightly lower Cmax in blood, but is insufficient to draw conclusions.
The two participants positive for MDMA, HMMA, and MDA after the placebo capsule (Online Resource 2) self-reported ingesting an average 8 MDMA pills/month in the prior 3 months (Table 1). Our closed research unit prevented drug intake within 12 h prior to controlled MDMA/placebo administration, and these participants self-reported last MDMA ingestion >16 h prior to the placebo dose. Although blood and plasma concentrations observed here did not result from controlled administration and should be interpreted with caution, they offer blood information from a longer post-dose window. The data collected from these individuals corroborates the MDMA and HMMA findings from controlled administration; blood concentrations were higher and lower than in plasma, respectively. MDA blood and plasma concentrations were near the LOQ throughout these sessions. Previous studies reported MDMA, HMMA, and MDA plasma t1/2 5.9–11.8 [10,7,11,33], 9.8–10.4 [7,33], and 9.3–17.7 h [10,7,33], respectively, with substantial t1/2 inter-subject variability [7]. Farre et al [10] documented longer MDMA and MDA t1/2 24–48 h after MDMA administration than in the first 24 h (8.8 vs. 7.0 and 14.1 vs. 12.8 h, respectively), indicating a longer secondary distribution and excretion phase.
Blood MDA/MDMA (p<0.0005) and MDA/HMMA (p<0.01) ratios significantly increased after dosing, while HMMA/MDMA significantly decreased (p<0.01), reaching a plateau after approximately 1.5 h (Fig. 3).The statistically significant (p=0.010) low-high MDA/MDMA dose difference observed at 3 h was minor and not clinically significant. Although initially unexpected that HMMA/MDMA decreased during the 3 h post-dose, this agrees with previous plasma findings [7] showing greater initial ratios that decreased for the first few hours post-dose as MDMA increased. HMMA/MDMA gradually increased during the 10–40 h post-dose window. Previous studies showed MDMA inhibits its own metabolism [7,10–11] through CYP2D6 inhibition of the primary pathway to HMMA. This inhibition occurs within 2 h [11]. By 4 h after 1.5 mg/kg MDMA, CYP1A2 activity increased 20–40% [34]. We hypothesize this process likely explains the patterns observed. As the major HMMA metabolic pathway was inhibited, the MDA pathway increased, thereby increasing MDA ratios. For the same reason, HMMA/MDMA was greater—and MDA/HMMA less—in the low dose relative to the high. Greater MDMA doses more rapidly inhibited the 2D6 enzyme.
Prior research [7] indicated median plasma tmax >3 h for MDA (7.1 h [low and high]) and HMA (11.0 h [low] and 12.0 h [high]). Our blood results appear to emulate those findings, since Cmax did not appear within the 3 h window. Similar observed tmax, occurring prior to 3 h, suggests true MDMA and HMMA Cmax are reported. AUC0–3h represents the MDMA absorption window, so our results matched the 1.6-fold dose difference. The nonlinear plasma pharmacokinetics we previously observed [7] appear to extend from inhibited metabolism and decreased clearance, causing lasting elevated concentrations that impacted the later time course. Our HMMA results for both matrices corroborate those previously described, showing equal Cmax and slightly-elevated (1.1 high/low ratio) AUC0–3h. The 1.3 previously-reported plasma AUC∞ ratio reflects longer-term metabolism inhibition. MDA patterns were similar, linearly reflecting the dose difference for this 3-h window despite showing greater differences in extended Cmax and AUC∞. HMA was detected in few blood specimens, corroborating a longer formation time. Extended plasma pharmacokinetics revealed 79% and 100% of 17 participants’ plasma specimens were HMA-positive 23 h after the low and high dose, respectively [7].
Between-subjects sex and race Cmax and AUC0–3h comparisons revealed significant differences after the low dose only, agreeing with our previous findings [7]. The greater blood MDMA Cmax in females (p=0.010) did not significantly alter AUC0–3h. We did not observe the previously-reported significantly increased female plasma MDA Cmax or AUC; but our decreased female HMMA low-dose AUC0–3h in whole blood and plasma supports previous plasma results. Females exhibit a stronger MDMA-induced CYP2D6 inhibition profile than males [35], possibly explaining decreased MDMA clearance after the lower dose only. We hypothesize the higher 1.6 mg/kg dose caused substantial enough enzyme inhibition that sex did not display the differential effect observed after the low dose.
Previously, we had too few participants to examine racial differences. In this study our sample was large enough that for the first time, we were able to directly compare and show a significant MDMA pharmacokinetic difference between African-American and white/Hispanic or mixed-race individuals. We observed a significantly lower (p=0.035) plasma HMMA AUC0–3h in African-American participants after the low dose, suggesting increased toxicity risk due to decreased metabolism. Although this was the only statistically-significant racial difference observed, it is possible that the diminished post-dose time course did not allow racial metabolic differences to fully manifest. As noted with sex differences, the observation that only the low dose showed a differential racial effect may indicate that the 1.6 mg/kg dose caused substantial enough enzyme inhibition that race did not display the differential effect observed after the low dose.
Genetic polymorphisms, particularly in the CYP2D6 enzyme, influence pharmacokinetic profiles of MDMA and its metabolites. A recent study by Pardo-Lozano et al [36] demonstrated that individuals carrying two functional alleles (FA) for CYP2D6 produce significantly higher HMMA plasma concentrations relative to carriers with one FA. It is likely that the patterns observed in plasma would be reflected in blood; i.e., higher blood HMMA concentrations (and possibly increased MDMA clearance) would be observed in cases of extensive metabolizers. However, we believe that MDMA inhibition of its own metabolism is a more important factor in explaining our findings, as also reported by Yubero-Lahoz et al [35]. We do not anticipate that this would alter blood vs. plasma findings.
In summary, we present for the first time blood MDMA and metabolites pharmacokinetics after controlled oral MDMA administration. The MDMA and metabolite blood/plasma correlation data presented here are highly relevant and, as far as we are aware, the first available in the literature. This is the first direct comparison between paired blood and plasma specimens, and the first evidence of racial differences in MDMA metabolism. The racial and sex differences we observed warrant further investigation in order to determine the extent of greater toxicity risk in females and African-Americans. These controlled MDMA administration data provide valuable information for interpretation of clinical and forensic data. This information fills a critical knowledge gap of MDMA and metabolites analysis and concentrations in blood; previous analytical procedures and results focused almost entirely on plasma. Our blood data and matrix comparisons will be beneficial for interpreting forensic cases, given the prior focus on plasma in the published literature.
Supplementary Material
Acknowledgements
We thank the National Institute on Drug Abuse Intramural Research Program and Johns Hopkins Bayview Medical Center Behavioral Pharmacology Research Unit clinical staff. We further acknowledge the Graduate Partnership Program, National Institutes of Health. This research was funded by the Intramural Research Program, National Institute on Drug Abuse, NIH.
References
- 1.Sumnall HR, Cole JC, Jerome L. The varieties of ecstatic experience: an exploration of the subjective experiences of ecstasy. J Psychopharmacol. 2006;20(5):670–682. doi: 10.1177/0269881106060764. [DOI] [PubMed] [Google Scholar]
- 2.de la Torre R, Farre M, Roset PN, Lopez CH, Mas M, Ortuno J, Menoyo E, Pizarro N, Segura J, Cami J. Pharmacology of MDMA in humans. Ann NY Acad Sci. 2000;914:225–237. doi: 10.1111/j.1749-6632.2000.tb05199.x. [DOI] [PubMed] [Google Scholar]
- 3.Downing J. The psychological and physiological effects of MDMA on normal volunteers. J Psychoactive Drugs. 1986;18:335–340. doi: 10.1080/02791072.1986.10472366. [DOI] [PubMed] [Google Scholar]
- 4.Cami J, Farre M, Mas M, Roset RN, Poudevida S, Mas A, San L, de la Torre R. Human pharmacology of 3,4-methylenedioxymethamphetamine (“Ecstasy”): psychomotor performance and subjective effects. J Clin Psychopharm. 2000;20(4):455–466. doi: 10.1097/00004714-200008000-00010. [DOI] [PubMed] [Google Scholar]
- 5.Lacey JH, Kelley-Baker T, Furr-Holden D, Voas RB, Romano E, Ramirez A, Brainard K, Moore C, Torres P, Berning A. 2007 National Roadside Survey of Alcohol and Drug Use by Drivers: Drug Results. National Highway Traffic Safety Administration Office of Behavioral Safety Research; 2009. [Accessed 15 Aug 2013]. http://www.nhtsa.gov/Driving+Safety/Research+&+Evaluation/2007+National+Roadside+Survey+of+Alcohol+and+Drug+Use+by+Drivers. [Google Scholar]
- 6.Substance Abuse and Mental Health Services Administration. Results from the 2009 National Survey on Drug Use and Health: National Findings NSDUH Series H-38A. Rockville, MD: Department of Health and Human Services (DHHS); 2010. [Accessed 15 Aug 2013]. http://www.samhsa.gov/data/2k9/2k9Resultsweb/web/2k9results.htm. [Google Scholar]
- 7.Kolbrich EA, Goodwin RS, Gorelick DA, Hayes RJ, Stein EA, Huestis MA. Plasma Pharmacokinetics of 3,4-Methylenedioxymethamphetamine After Controlled Oral Administration to Young Adults. Ther Drug Monit. 2008;30(3):320–332. doi: 10.1097/FTD.0b013e3181684fa0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meyer MR, Peters FT, Maurer HH. The role of human hepatic cytochrome P450 isozymes in the metabolism of racemic 3,4-methylenedioxy-methamphetamine and its enantiomers. Drug Metab Dispos. 2008;36(11):2345–2354. doi: 10.1124/dmd.108.021543. [DOI] [PubMed] [Google Scholar]
- 9.Kolbrich EA, Goodwin RS, Gorelick DA, Hayes RJ, Stein EA, Huestis MA. Physiological and subjective responses to controlled oral 3,4-methylenedioxymethamphetamine administration. J Clin Psychopharmacol. 2008;28(4):432–440. doi: 10.1097/JCP.0b013e31817ef470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farre M, de la Torre R, Mathuna BO, Roset PN, Peiro AM, Torrens M, Ortuno J, Pujadas M, Cami J. Repeated doses administration of MDMA in humans: pharmacological effects and pharmacokinetics. Psychopharmacology. 2004;173(3–4):364–375. doi: 10.1007/s00213-004-1789-7. [DOI] [PubMed] [Google Scholar]
- 11.Peiro AM, Farre M, Roset PN, Carbo M, Pujadas M, Torrens M, Cami J, de la Torre R. Human pharmacology of 3,4-methylenedioxymethamphetamine (MDMA, ecstasy) after repeated doses taken 2 h apart. Psychopharmacology. 2013;225(4):883–893. doi: 10.1007/s00213-012-2894-7. [DOI] [PubMed] [Google Scholar]
- 12.Ramaekers JG, Kuypers KP, Samyn N. Stimulant effects of 3,4-methylenedioxymethamphetamine (MDMA) 75 mg and methylphenidate 20 mg on actual driving during intoxication and withdrawal. Addiction. 2006;101(11):1614–1621. doi: 10.1111/j.1360-0443.2006.01566.x. [DOI] [PubMed] [Google Scholar]
- 13.Pizarro N, Farre M, Pujadas M, Peiro AM, Roset PN, Joglar J, de la Torre R. Stereochemical analysis of 3,4-methylenedioxymethamphetamine and its main metabolites in human samples including the catechol-type metabolite (3,4-dihydroxymethamphetamine) Drug Metab Dispos. 2004;32(9):1001–1007. [PubMed] [Google Scholar]
- 14.Mueller M, Peters FT, Huestis MA, Ricaurte GA, Maurer HH. Simultaneous liquid chromatographic-electrospray ionization mass spectrometric quantification of 3,4-methylenedioxymethamphetamine (MDMA, Ecstasy) and its metabolites 3,4-dihydroxymethamphetamine, 4-hydroxy-3-methoxymethamphetamine and 3,4-methylenedioxyamphetamine in squirrel monkey and human plasma after acidic conjugate cleavage. Forensic Sci Int. 2009;184(1–3):64–68. doi: 10.1016/j.forsciint.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peters FT, Samyn N, Lamers CT, Riedel WJ, Kraemer T, de Boeck G, Maurer HH. Drug testing in blood: validated negative-ion chemical ionization gas chromatographic-mass spectrometric assay for enantioselective measurement of the designer drugs MDEA, MDMA, and MDA and its application to samples from a controlled study with MDMA. Clin Chem. 2005;51(10):1811–1822. doi: 10.1373/clinchem.2005.052746. [DOI] [PubMed] [Google Scholar]
- 16.Desrosiers NA, Barnes AJ, Hartman RL, Scheidweiler KB, Kolbrich-Spargo EA, Gorelick DA, Goodwin RS, Huestis MA. Oral fluid and plasma 3,4-methylenedioxymethamphetamine (MDMA) and metabolite correlation after controlled oral MDMA administration. Anal Bioanal Chem. 2013;405(12):4067–4076. doi: 10.1007/s00216-013-6848-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kolbrich EA, Lowe RH, Huestis MA. Two-Dimensional Gas Chromatography/Electron-Impact Mass Spectrometry with Cryofocusing for Simultaneous Quantification of MDMA, MDA, HMMA, HMA, and MDEA in Human Plasma. Clin Chem. 2008;54:379–387. doi: 10.1373/clinchem.2007.096800. [DOI] [PubMed] [Google Scholar]
- 18.Scientific Working Group for Forensic Toxicology (SWGTOX) [Acessed 15 Aug 2013];Standard Practices for Method Validation in Forensic Toxicology. 2013 doi: 10.1093/jat/bkt054. http://www.swgtox.org/documents/Validation3.pdf. [DOI] [PubMed]
- 19.Scheidweiler KB, Barnes AJ, Huestis MA. A validated gas chromatographic-electron impact ionization mass spectrometric method for methamphetamine, methylenedioxymethamphetamine (MDMA), and metabolites in mouse plasma and brain. J Chromatogr B. 2008;876(2):266–276. doi: 10.1016/j.jchromb.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Helmlin H, Bracher K, Bourquin D, Vonlanthen D, Brenneisen R. Analysis of 3,4-methylenedioxymethamphetamine (MDMA) and its metabolites in plasma and urine by HPLC-DAD and GC-MS. J Anal Toxicol. 1996;20:432–440. doi: 10.1093/jat/20.6.432. [DOI] [PubMed] [Google Scholar]
- 21.Clauwaert KM, Van Bocxlaer JF, DeLetter EA, Van Calenbergh S, Lambert WE, De Leenheer AP. Determination of the designer drugs 3,4-methylenedioxymethamphetamine, 3,4-methylenedioxyethylamphatamine, and 3,4-methylenedioxyamphetamine with HPLC and fluorescence detection in whole blood, serum, vitreous humor, and urine. Clin Chem. 2000;46:1968–1977. [PubMed] [Google Scholar]
- 22.Cheze M, Deveaux M, Martin C, Lhermitte M, Pepin G. Simultaneous analysis of six amphetamines and analogues in hair, blood and urine by LC-ESI-MS/MS. Application to the determination of MDMA after low ecstasy intake. Forensic Sci Int. 2007;170(2–3):100–104. doi: 10.1016/j.forsciint.2007.02.033. [DOI] [PubMed] [Google Scholar]
- 23.Middleberg RA, Homan J. Quantitation of amphetamine-type stimulants by LC-MS/MS. Methods Mol Biol. 2012;902:105–114. doi: 10.1007/978-1-61779-934-1_9. [DOI] [PubMed] [Google Scholar]
- 24.Bjork MK, Nielsen MK, Markussen LO, Klinke HB, Linnet K. Determination of 19 drugs of abuse and metabolites in whole blood by high-performance liquid chromatography-tandem mass spectrometry. Anal Bioanal Chem. 2010;396(7):2393–2401. doi: 10.1007/s00216-009-3268-9. [DOI] [PubMed] [Google Scholar]
- 25.da Silva DG, de Pinho PG, Pontes H, Ferreira L, Branco P, Remiao F, Carvalho F, Bastos ML, Carmo H. Gas chromatography-ion trap mass spectrometry method for the simultaneous measurement of MDMA (ecstasy) and its metabolites, MDA, HMA, and HMMA in plasma and urine. J Chromatogr B. 2010;878(9–10):815–822. doi: 10.1016/j.jchromb.2010.01.042. [DOI] [PubMed] [Google Scholar]
- 26.Tomita M, Nakashima MN, Wada M, Nakashima K. Sensitive determination of MDMA and its metabolite MDA in rat blood and brain microdialysates by HPLC with fluorescence detection. Biomed Chromatogr. 2007;21(10):1016–1022. doi: 10.1002/bmc.839. [DOI] [PubMed] [Google Scholar]
- 27.Jantos R, Veldstra JL, Mattern R, Brookhuis KA, Skopp G. Analysis of 3,4-methylenedioxymetamphetamine: whole blood versus dried blood spots. J Anal Toxicol. 2011;35(5):269–273. doi: 10.1093/anatox/35.5.269. [DOI] [PubMed] [Google Scholar]
- 28.Navarro M, Pichini S, Farre M, Ortuno J, Roset PN, Segura J, de La Torre R. Usefulness of saliva for measurement of 3,4-methylenedioxymehamphetamine and its metabolites: correlation with plasma drug concentrations and effect of salivary pH. Clin Chem. 2001;47:1788–1795. [PubMed] [Google Scholar]
- 29.Moffat AC, Osselton MD, Widdop B, Galichet LY. Clarke's analysis of drugs and poisons in pharmaceuticals, body fluids and postmortem material. 3rd edn. London: Pharmaceutical Press; 2004. [Google Scholar]
- 30.Clauwaert KM, Van Bocxlaer JF, De Leenheer AP. Stability study of the designer drugs 'MDA, MDMA and MDEA' in water, serum, whole blood, and urine under various storage temperatures. Forensic Sci Int. 2001;124:36–42. doi: 10.1016/s0379-0738(01)00562-x. [DOI] [PubMed] [Google Scholar]
- 31.Garrett ER, Seyda K, Marroum P. High performance liquid chromatographic assays of the illicit designer drug 'Ecstasy', a modified amphetamine, with applications to stability, partitioning and plasma protein binding. Acta Pharm Nord. 1991;3(1):9–14. [PubMed] [Google Scholar]
- 32.Belhadj-Tahar H, Payoux P, Tafani M, Coulais Y, Calet S, Bousseksou A. Toxicological methods for tracing drug abuse: chromatographic, spectroscopic and biological characterisation of ecstasy derivatives. Arh Hig Rada Toksikol. 2010;61(1):53–59. doi: 10.2478/10004-1254-61-2010-1937. [DOI] [PubMed] [Google Scholar]
- 33.Capela JP, Carmo H, Remiao F, Bastos ML, Meisel A, Carvalho F. Molecular and cellular mechanisms of ecstasy-induced neurotoxicity: an overview. Mol Neurobiol. 2009;39(3):210–271. doi: 10.1007/s12035-009-8064-1. [DOI] [PubMed] [Google Scholar]
- 34.Yubero-Lahoz S, Pardo R, Farre M, Mathuna BO, Torrens M, Mustata C, Perez-Mana C, Langohr K, Carbo ML, de la Torre R. Changes in CYP1A2 activity in humans after 3,4-methylenedioxymethamphetamine (MDMA, ecstasy) administration using caffeine as a probe drug. Drug Metab Pharmacokinet. 2012;27(6):605–613. doi: 10.2133/dmpk.dmpk-12-rg-032. [DOI] [PubMed] [Google Scholar]
- 35.Yubero-Lahoz S, Pardo R, Farre M, O'Mahony B, Torrens M, Mustata C, Perez-Mana C, Carbo ML, de la Torre R. Sex differences in 3,4-methylenedioxymethamphetamine (MDMA; ecstasy)-induced cytochrome P450 2D6 inhibition in humans. Clin Pharmacokinet. 2011;50(5):319–329. doi: 10.2165/11584550-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 36.Pardo-Lozano R, Farré M, Yubero-Lahoz S, O'Mathúna B, Torrens M, Mustata C, Pérez-Mañá C, Langohr K, Cuyàs E, Carbó Ml, de la Torre R. Clinical pharmacology of 3,4-methylenedioxymethamphetamine (MDMA, 'ecstasy'): the influence of gender and genetics (CYP2D6, COMT, 5-HTT) PLoS One. 2012;7(10):e47599. doi: 10.1371/journal.pone.0047599. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.