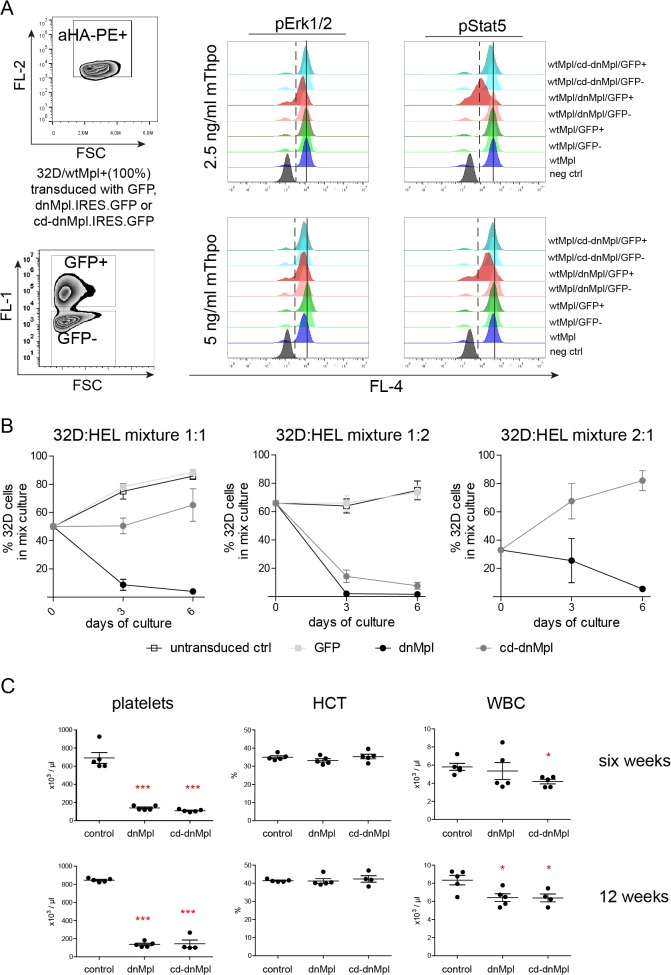
Fig 3. Systemic and cell intrinsic effects of dnMpl expression.
(A) wtMpl expressing 32D cells (aHA-PE positive) were transduced with dnMpl.IRES.GFP, constitutive dimerized (cd)-dnMpl.IRES.GFP or GFP encoding vectors to establish cultures with single and double positive cells. Cells were starved for any cytokine stimuli for 16 hrs and stimulated with 2.5 or 5 ng/mL mThpo for 15 minutes the next day. Unstimulated (negative control) and stimulated cells were fixed and permeabelized to allow intracellular staining of phosphorylated signaling molecules. Anti-phosphoERK1/2 or phosphoSTAT5 antibodies conjugated to Alexa Fluor 647 (BD Biosciences) were used. Shown are histogram overlays of pERK1/2 and pSTAT5 activation from wtMpl/GFP negative cells and wtMpl/GFP, wtMpl/dnMpl, wtMpl/cd-dnMpl double positive cells. (Dashed line—activation border; solid line—mean fluorescence intensity of wtMpl/GFP double positive cells). (B) Human erythroid leukemia (HEL) cells were transduced with retroviral vectors encoding dnMpl, cd-dnMpl or GFP as control. wtMpl expressing 32D cells (100% positive) were mixed with untransduced, GFP control, dnMpl or cd-dnMpl expressing HEL cells in different ratios (1:1, 1:2, 2:1). Cells were co-cultured in murine Thpo supplemented medium (6ng/mL). The percentage of 32D cells three and six days after co-culture was measured. In the absence of dnMpl or cd-dnMpl wtMpl/32D cells grew faster than HEL cells. But in the presence of dnMpl (1:1, 1:2 and 2:1) or cd-dnMpl (1:2) wtMpl/32D cells stopped proliferating and were diminished over time in the culture. (C) Blood counts of mice transplanted with dnMpl, cd-dnMpl or GFP control transduced wildtype Lin- BM cells six and twelve weeks after transplantation (*p<0.05, ***p<0.005 Student’s t-test, n = 4–5). One of the cd-dnMpl mice had to be killed at 12 weeks due to the severe anemia.