Figure 10.
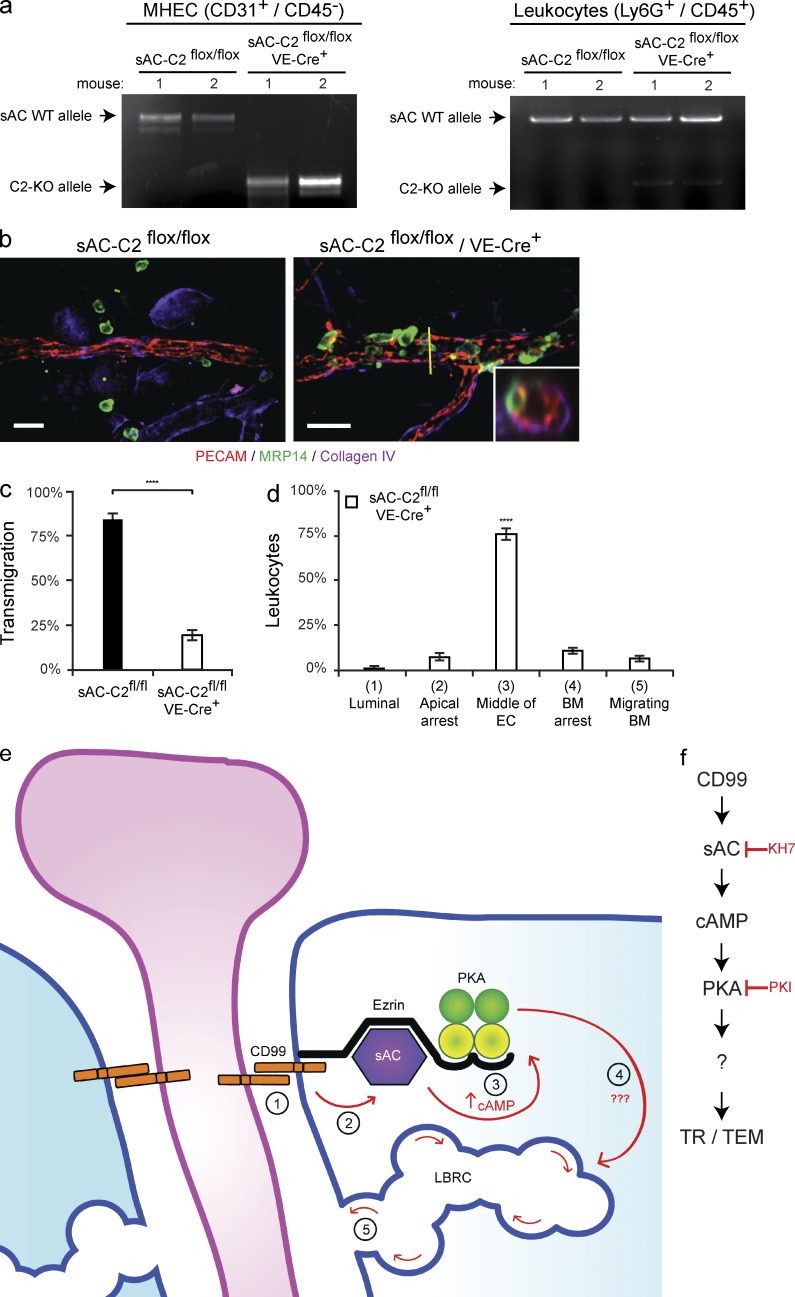
Endothelial-specific knockout of sAC blocks leukocyte transmigration in vivo. (a) Heart tissue and peripheral blood was collected from sAC-C2flox/flox and sAC-C2flox/flox/VE-Cre+ mice used in the following experiments (see Materials and methods). FACS was used to sort MHEC (CD31+/CD45−) and leukocyte (Ly6G+/CD45+) cell populations from the heart tissue and peripheral blood, respectively. MHEC and leukocyte DNA was isolated from each mouse. PCR was performed to assess the expression of either sAC WT allele (top band) or sAC C2-KO allele (bottom band). (b) The ears of sAC-C2flox/flox or sAC-c2flox/flox/VE-Cre+ mice (mixed background, see Materials and methods; Chen et al., 2013) were stimulated for 5 h with croton oil. Tissue was then harvested, stained, and analyzed. (c) Quantification of results above. (d) Quantification of site of arrest for sAC-C2flox/flox/VE-Cre+ mice. Percent of leukocytes extravasated within 50 µm of venule per field of view. (e) Our current model of how CD99 signals during TEM. Under resting conditions, CD99, sAC, PKA, and ezrin form a signaling complex at endothelial junctions. During TEM, homophilic engagement of endothelial CD99 with leukocyte CD99 (#1) signals through sAC (#2) to elevate cAMP (#3), to activate PKA, which works through a yet to be defined mechanism (#4) to induce LBRC membrane trafficking to sites of leukocyte–endothelial contact (#5). (f) Flow diagram of model detailed above. 100–200 cells were analyzed per ear. PMN per field of view, vessel length and vessel diameter were equivalent for all conditions tested (not depicted). Images were acquired with a 40× objective (n = 1.00). Insets show xz-orthogonal view (where yellow bar dissects the vessel) to demonstrate site of neutrophil arrest. Bars, 25 µm. Three mice per condition were used for each experiment. Images are representative of three (a and b) independent experiments. Data represent the average value of three (c and d) independent experiments. Error bars denote SEM (****, P < 0.0001; Student’s t test [c] and ANOVA [d]).