Extended Data Figure 1.
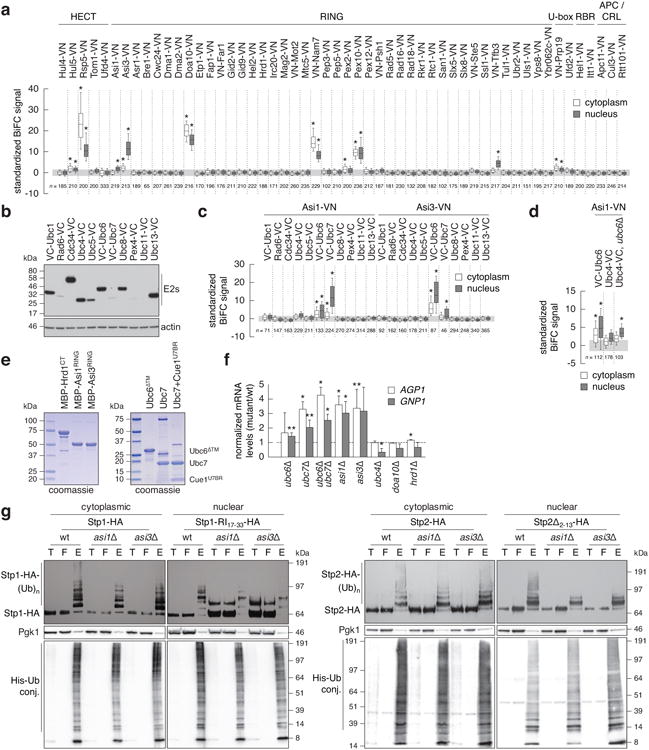
Identification of Ubc6 and Ubc7 ubiquitin conjugating enzymes as functional interacting partners of Asi1 and Asi3.
a, Quantification of BiFC signals in cells expressing VC-Ubc6 and all tested E3 ubiquitin ligases. BiFC signals were measured in the cytoplasm and nucleus of individual cells (n as indicated in the figure). Whiskers extend from 10th to 90th percentiles. The same representation is used in c and d.
b, Immunoblot showing expression levels of VC-tagged E2 ubiquitin conjugating enzymes. Ubc11-VC could not be detected in the growth condition of the BiFC assay.
c, Quantification of BiFC signals in cells co-expressing VC-tagged E2 ubiquitin conjugating enzymesand Asi1-VN or Asi3-VN (n as indicated in the figure).
d, Detection of a significant BiFC signal between Asi1-VN and Ubc4-VCin cells lacking UBC6 (n as indicated in the figure).
e, Coomassie-stained gels of recombinant proteins used in microscale thermophoresis experiments.
f, mRNA levels of AGP1 and GNP1 measured with qRT-PCR in the indicated strains (mean ± s.d., n = 3 clones). The signal was normalized to wild type (dashed line).
g, Ubiquitylation of Stp1-HA or Stp1-RI17-33-HA (left panel) and Stp2-HA or Stp2Δ2-13-HA (right panel) in strains expressing 6His-ubiquitin. Stp1-RI17-33 and Stp2Δ2-13 variants exhibit compromised cytoplasmic retention and enhanced Asi-dependent degradation, whereas full length Stp1 is degraded primarily in the cytoplasm in SCFGrr1-dependent manner11. Total cell extracts (T), flow through (F) and ubiquitin conjugates (E) eluted after immobilized-metal affinity chromatography were separated by SDS-PAGE followed by immunoblotting with antibodies against the HA tag, Pgk1 and the His tag. Representative immunoblots from 3 technical replicates.
Statistics: (a, c, d) One-way ANOVA with Bonferroni correction for multiple testing. *P<10-4. (f) Two-tailed t-test. *P<0.05 **P<0.10.
