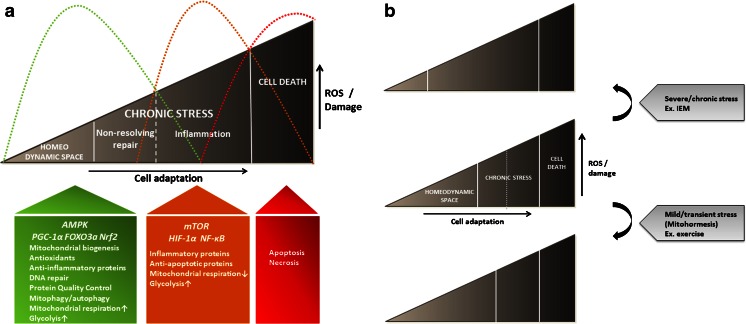
Fig. 3.
The ROS triangle model links increasing ROS and damage to chronic stress adaptation with non-resolving repair responses (green graphic), or compromised repair responses that drive a more pro-inflammatory environment (orange graphic). The antagonistic cell stress responses are linked to distinct cellular metabolism through redox-sensitive nutrient-sensing signalling growth pathways that control a Warburg-like shift from mitochondrial respiration (AMPK/PGC-1α) towards mostly cytosolic glycolysis (mTOR/HIF-1α). ROS, but also NAD+, are the most important mitochondrial signalling molecules that drive the transition from one stage of the triangle to another as discussed in the text and illustrated in Fig. 4. When oxidative stress becomes too high to allow cell stress adaptive redox signalling, apoptosis and cell death are induced (red graphic) (a). In the sick cell, chronic non-resolving repair responses or inflammatory responses will dominate depending on the duration and/or level of ROS load. In the healthy cell, well-controlled physiological levels of ROS allow healthy redox signalling and dynamic cell stress responses to ensure that inflammatory and damaging cell responses are followed by repair responses to restore homeostasis. The ROS range, at which dynamic healthy redox signalling is taking place, to regulate transient physiological changes or stressors like cell growth/differentiation and inflammation/repair, is called the homeodynamic space. Mild and transient oxidative stress, such as exercise and caloric restriction, increases the homeodynamic space by boosting repair responses, and prevent chronic disease development. IEM and other persistent ROS-inducers decrease the homeodynamic space, making the cells more prone to chronic disease development (b)