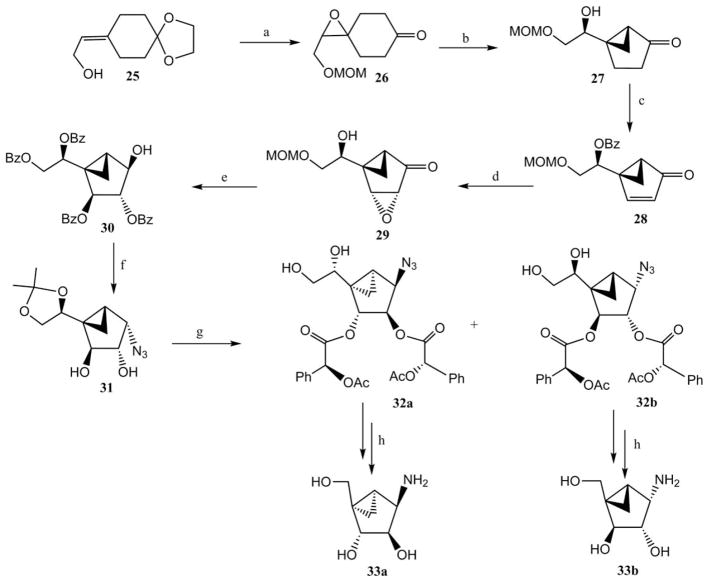
Scheme 4.
Novel synthetic route to the (N)-methanocarba equivalents of β-arabinofuranosyl and α-galactofuranosyl 1-amino sugars.23 Reagents and conditions: (a) (i) oxalic acid, acetone–H2O; (ii) (MeO)2CH2, LiBr, p-TsOH; (iii) m-CPBA; (b) NaOH, EtOH; (c) (i) BzOH, Ph3P, DIAD; (ii) PhSeCl, HCl; (iii) NaIO4; (d)H2O2, NaOH; (e) (i) H2SO4, (ii) BzCl, pyridine; (iii) NaBH4; (f) (i) (PhO)2PON3, Ph3P, DIAD; (ii) MeONa, MeOH; (iii) (MeO)2CMe2, p-TsOH; (g) (i) O-acetyl-(S)-mandelic acid, DCC, DMP; (ii) AcOH–H2O; (h) (i) NaIO4; (ii) MeONa; (iii) NaBH4; (iv) H2, Pd/C.