Fig. 6.
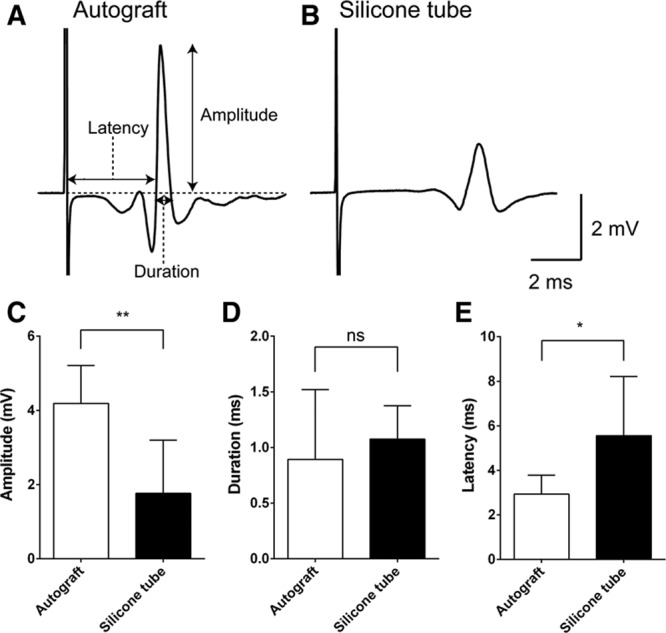
CMAP analysis. For recording CMAP, a stainless steel microelectrode (9–12 MΩ at 1 kHz) (Uesmgcselnnm-type) (FHC, Bowdoin, Maine) was inserted into the vibrissal muscles between the middle vibrissal rows C and D. A reference electrode (TN204-089B) (Unique Medical, Tokyo, Japan) was placed in the caudal position of the skull. For stimulating the regenerated facial nerve, a tandem hook-shaped stimulation electrode (IMC-220224) (InterMedical, Aichi, Japan) was connected to the exfoliated nerve of the buccal branch of facial nerve in the autograft (n = 3) and silicone tube groups (n = 4), and stimulation pulses at a supramaximal level of 2 mA (100 μs monopolar pulses) were delivered at 0.2 Hz via an isolator (SS-202J) (Nihon Kohden, Tokyo, Japan). Recorded signals were processed with a multichannel amplifier (MEG-6100) (Nihon Kohden) at 15–10,000 Hz and digitized at 40 kHz using a PowerLab4/30 and LabChart7 system (ADInstruments, Dunedin, New Zealand). Data were analyzed in an off-line manner with Igor Pro software (Wavemetrics, Lake Oswego, Oreg.). The upward trace of CMAP gave a negative deflection (depolarization), and 10 consecutive traces were averaged. CMAP traces recorded from a whisker pad after supramaximal stimulation IPJG with autograft (A) and in silicone tube (B). Although amplitude (C) values were significantly larger in the autograft group (4.19 ± 1.02 mV) (n = 3) than those in the silicone tube group (1.76 ± 1.43 mV) (n = 4), latency (E) values were significantly lower in the autograft graft group (2.93 ± 0.85 ms) (n = 3) than those in the silicone tube group (5.56 ± 2.65 ms) (n = 4). D, No significant difference in duration was observed (0.89 ± 0.63 ms vs 1.08 ± 0.30 ms). CMAP amplitude, duration, and latency were compared by unpaired t test. *P < 0.05. **P < 0.01. ns indicates not significant.
