Summary:
We report 3 cases of breast pyoderma gangrenosum in patients undergoing total mastectomy with immediate reconstruction. All three received systemic corticosteroid treatment, resulting in resolution of symptoms. As experience grew, early diagnosis in the third patient helped prosthesis salvage and timely return to the original course of reconstruction. This represents the first report of prosthesis salvage from post breast reconstruction pyoderma gangrenosum, and it demonstrates that implant removal is not always necessary during management of this rare condition.
Pyoderma gangrenosum (PG) is a noninfectious ulcerative dermatosis, histologically characterized by neutrophilic inflammation and infiltration.1,2 Fifty to seventy percent of PG is associated with systemic inflammatory disease or hematological malignancy.3 In breast reconstruction, diagnosis can be delayed because the clinical presentation mimics an infectious process, which prompts antibiotic therapy, serial debridement, and in cases of prosthetic reconstruction, implant removal, leaving the patient with extensive scarring and need for further breast reconstruction.4–6 Immunosuppression with corticosteroids is the mainstay of treatment of PG.7 Postsurgical PG of the breast is not common, with 22 cases reported following breast reductions and pexies, 7 after breast reconstructions, and 2 following breast augmentations.8
We present 3 cases of PG following total mastectomy with immediate reconstruction surgery and highlight 1 case of PG, as experience grew, where timely diagnosis and appropriate treatment resulted in implant salvage and return to the original course of reconstruction.
CASE 1
A 62-year-old white woman had bilateral total mastectomy and immediate reconstruction with bilateral pedicle transverse rectus abdominis myocutaneous flaps. Postoperatively, the patient was doing well initially, with no skin edge necrosis of breast skin. On postoperative day (POD) 4, she had mild erythema involving both native breast skin, more on the right than left. She had no fever and no chills; routine blood work was negative. The patient was discharged with advice to follow up in a week. However, on POD 9, the patient presented with severe inflammation of both breast skin flaps, with the right side worse than left and no involvement of the transverse rectus abdominis myocutaneous flaps. There were multiple yellow dermal pustules among the skin ulceration. The patient was taken to the operating room, where the involved skin was conservatively debrided. Postoperatively, the patient was started on oral prednisolone (1 mg/kg), and pathology was consistent with the clinical diagnosis of PG. Further local wound therapy involving vacuum assisted closure device and eventual skin graft resulted in fully healed operative sites with the expected scarring. Bilateral nipple reconstruction along with minor revision was done 3 months later. There was no recurrence of symptoms during follow-ups.
CASE 2
A 56-year-old white woman underwent right total mastectomy with sentinel node biopsy and immediate breast reconstruction with a tissue expander (Allergan, Irvine, Calif.) with AlloDerm (LifeCell, Branchburg, N.J.). Postoperative period was uneventful with discharge on POD 2. At the time of discharge, the surgical site was clean and healing well. The patient presented to the clinic on POD 8 with pain and inflammation over her breast. Peri-incisional erythema, necrosis with punctate yellow dermal pustules, and purulent fluid were noted. There was no fever, chills, or expansion of the erythema.
A diagnosis of PG was tentatively made by a dermatology consultant, and the patient was started on a systemic corticosteroid (oral prednisolone, 1 mg/kg/d). She was taken to the operating room for surgical debridement of the affected skin that necessitated implant removal for wound closure purposes. The patient had complete resolution of symptoms and healing of the wound. Pathology was consistent with the clinical diagnosis of PG. Steroid therapy was gradually tapered off over 9 weeks. At 5-month follow-up, the patient was doing well, and there was no recurrence of symptoms. She was contemplating delayed breast reconstruction.
CASE 3
A 64-year-old white woman with no prior history of systemic inflammatory disease underwent right total mastectomy with sentinel node biopsy indicated for a poorly differentiated invasive ductal carcinoma with ductal carcinoma in situ grade 3 comedo necrosis. Immediate breast reconstruction was performed using a tissue expander and AlloDerm sling. Her postoperative course was remarkable for a 1-mm rim of suture line skin edge necrosis with intact blistering that was treated with Silvadene dressing (Pfizer, Cambridge, Mass.) changes. She was discharged home on POD 3.
On POD 9, the patient presented to the emergency department with chest discomfort and increasing erythema of the right breast associated with expanded area of superficial ulcerations. She was admitted for presumed cellulitis and intravenous antibiotics were administered. Progression of the ulcerations was noted despite systemic antibiotic therapy. Based on previous experience and the appearance of dermal yellow pustules within the ulcerations (Fig. 1), a diagnosis of PG was suspected. The patient was started on oral prednisolone (1 mg/kg/d), and subsequent lack of progression with notable improvement in appearance of the ulcerations strongly suggested a diagnosis of PG.
Fig. 1.
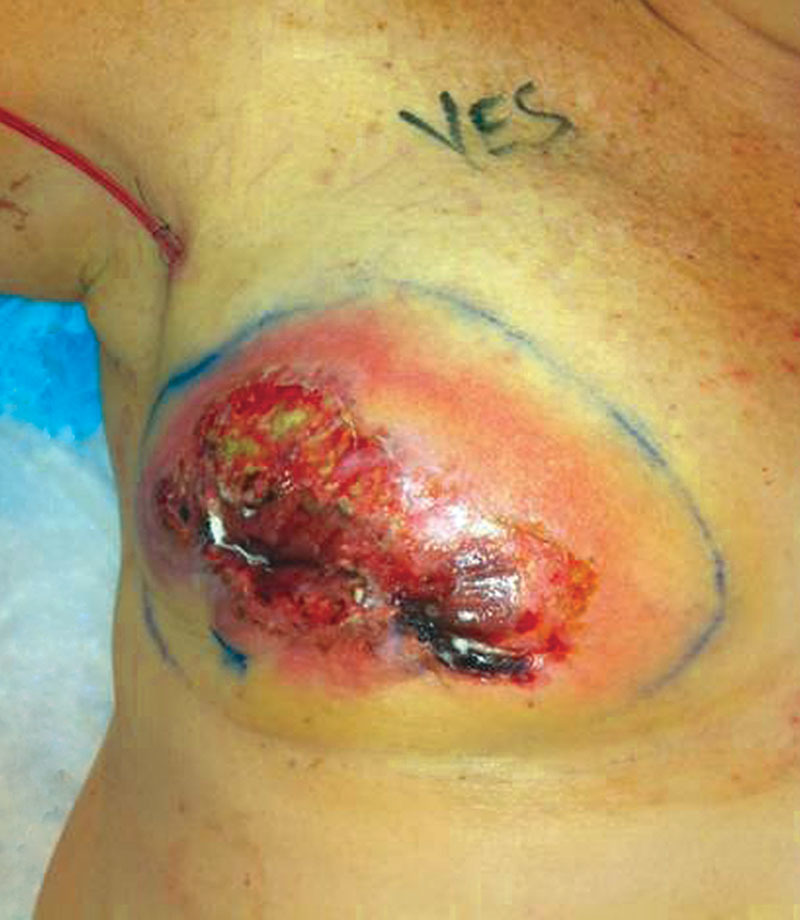
A diagnosis of postsurgical pyoderma gangrenosum should be considered in cases of appearance of dermal yellow pustules with ulcerations that do not respond to antibiotic therapy at postoperative days 3–5.
She also had skin debridement on POD 10 (Fig. 2). On exploration, suture line skin edge necrosis was resected along with the surrounding ulcerative dermal tissue. There was no purulent drainage, fat necrosis, or fluid collection identified. Copious irrigation with antibiotic solution preceded partial tissue expander deflation to facilitate skin edge reapproximation, leaving the original tissue expander and Jackson-Pratt drain (Cardinal Health, Dublin, Ohio) in place. Histology of the dermal ulcerative speci men was consistent with the clinical diagnosis of PG (Fig. 3). No bacterial growth was found in specimens.
Fig. 2.

Ulcerations seen on POD 10. On exploration, suture line skin edge necrosis was resected along with the surrounding ulcerative dermal tissue. There was no purulent drainage, fat necrosis, or fluid collection identified. Copious irrigation with antibiotic solution preceded partial tissue expander deflation to facilitate skin edge reapproximation, leaving the original tissue expander and Jackson-Pratt drain in place.
Fig. 3.
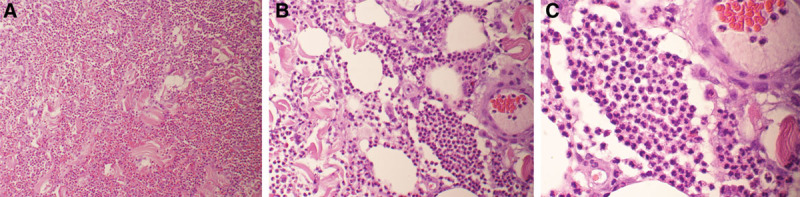
A–C, Histology of progressively higher powers of the dermal ulcerative specimen was consistent with the clinical diagnosis of pyoderma gangrenosum.
The patient’s postoperative course was unremarkable, and she was discharged home on the fifth day on a prednisone taper. Placement of permanent silicone prosthesis was done 3 months after initial reconstruction without further complications.
DISCUSSION
Diagnosis of PG is challenging, as clinical signs closely resemble an infectious process with no diagnostic laboratory or pathognomic histological findings. PG is a diagnosis of exclusion based on clinical factors, ulcerative characteristics, failure to respond to antibiotic and surgical therapy, and improvement in response to steroid treatment.9,10
In all 3 of our cases, symptoms of PG presented around POD 3–4, with dramatic worsening of the superficial skin ulceration just beyond a week postoperatively. This presentation course may aid the diagnosis of the disease, as pure skin edge necrosis becomes evident shortly after the surgery and postoperative infection usually does not manifest significantly until after a week.
CONCLUSION
Early diagnosis of PG is vital because it may help in preventing secondary infection of the wound and increases the likelihood of implant salvage and less aggressive subsequent reconstructive surgeries.
Footnotes
Drs. Cicuto and Cheriyan contributed equally to this work.
Disclosure: The authors have no financial interest to declare in relation to the content of this article. The Article Processing Charge was paid for by the authors.
REFERENCES
- 1.Duval A, Boissel N, Servant JM, et al. Pyoderma gangrenosum of the breast: a diagnosis not to be missed. J Plast Reconstr Aesthet Surg. 2011;64:e17–e20. doi: 10.1016/j.bjps.2010.07.022. [DOI] [PubMed] [Google Scholar]
- 2.Segaran A, Mohammad M, Sterling JC, et al. Pyoderma gangrenosum arising in a breast reduction scar: seven years post-procedure. J Plast Reconstr Aesthet Surg. 2013;66:e370–e372. doi: 10.1016/j.bjps.2013.05.016. [DOI] [PubMed] [Google Scholar]
- 3.Wallach D, Vignon-Pennamen MD. From acute febrile neutrophilic dermatosis to neutrophilic disease: forty years of clinical research. J Am Acad Dermatol. 2006;55:1066–1071. doi: 10.1016/j.jaad.2006.07.016. [DOI] [PubMed] [Google Scholar]
- 4.MacKenzie D, Moiemen N, Frame JD. Pyoderma gangrenosum following breast reconstruction. Br J Plast Surg. 2000;53:441–443. doi: 10.1054/bjps.2000.3349. [DOI] [PubMed] [Google Scholar]
- 5.Bonamigo RR, Behar PR, Beller C, et al. Pyoderma gangrenosum after silicone prosthesis implant in the breasts and facial plastic surgery. Int J Dermatol. 2008;47:289–291. doi: 10.1111/j.1365-4632.2008.03480.x. [DOI] [PubMed] [Google Scholar]
- 6.Rietjens M, Cuccia G, Brenelli F, et al. A pyoderma gangrenosum following breast reconstruction: a rare cause of skin necrosis. Breast J. 2010;16:200–202. doi: 10.1111/j.1524-4741.2009.00887.x. [DOI] [PubMed] [Google Scholar]
- 7.Brooklyn T, Dunnill G, Probert C. Diagnosis and treatment of pyoderma gangrenosum. BMJ. 2006;333:181–184. doi: 10.1136/bmj.333.7560.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grillo MA, Cavalheiro TT, da Silva Mulazani M, et al. Postsurgical pyoderma gangrenosum complicating reduction mammaplasty. Aesthetic Plast Surg. 2012;36:1347–1352. doi: 10.1007/s00266-012-9981-3. [DOI] [PubMed] [Google Scholar]
- 9.Mansur AT, Balaban D, Göktay F, et al. Pyoderma gangrenosum on the breast: a case presentation and review of the published work. J Dermatol. 2010;37:107–110. doi: 10.1111/j.1346-8138.2009.00756.x. [DOI] [PubMed] [Google Scholar]
- 10.Su WP, Davis MD, Weenig RH, et al. Pyoderma gangrenosum: clinicopathologic correlation and proposed diagnostic criteria. Int J Dermatol. 2004;43:790–800. doi: 10.1111/j.1365-4632.2004.02128.x. [DOI] [PubMed] [Google Scholar]


