Figure 3.
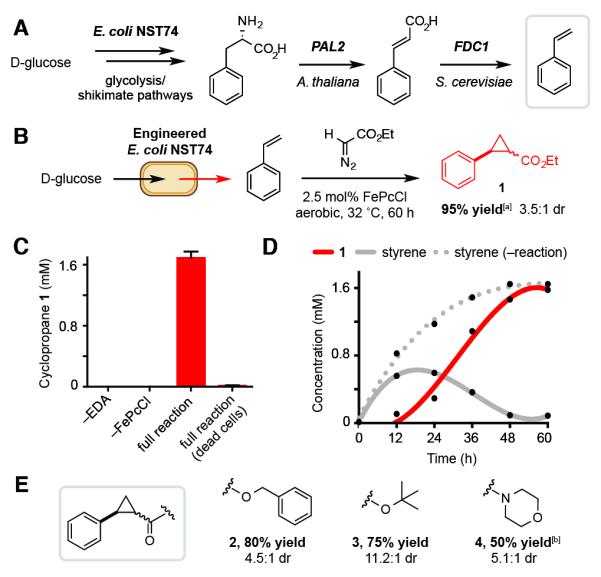
The biocompatible cyclopropanation reaction can be interfaced with microbial styrene production. A) Engineered pathway for styrene production in the L-phenylalanine overproducer E. coli NST74. B) Cyclopropanation using metabolically generated styrene. C) Cyclopropane production requires all reaction components and living E. coli. D) Metabolite production during fermentations. E) Additional cyclopropanes accessed via this approach. Metabolite concentrations were determined by GC relative to an internal standard of 1,3,5-trimethoxybenzene. Yields in Section E are of isolated material from 800 mL cultures. All data is shown as an average of three independent experiments to one standard deviation. [a] 93% isolated yield. [b] 72 h reaction.
