Table 3.
In vitro antimalarial activity of prodiginines containing aryl substituents at either the 3, or the 3 and 5 positions, of the terminal pyrrole ring
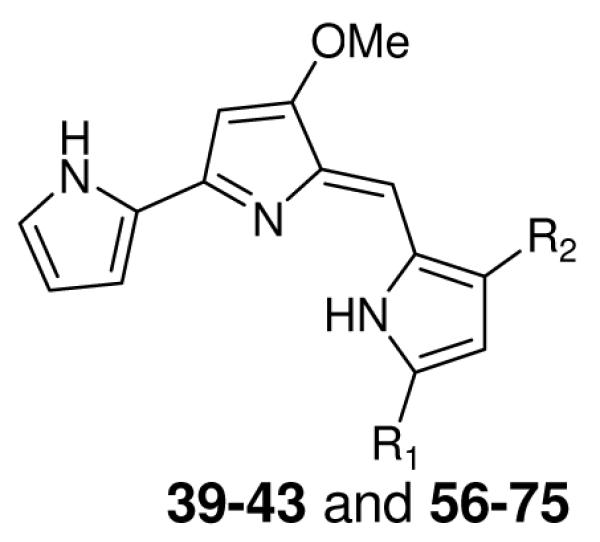
| |||||
|---|---|---|---|---|---|
|
| |||||
| Compd | R1 | R2 | IC50 (nM) |
Cytotoxicity (nM) |
|
| D6 | Dd2 | ||||
| CQ | - | - | 11 | 135 | 16057 |
| 39 | H | C6H5CH2 | 83 | 86 | < 125 |
| 40 | H | 4-OCH3C6H4CH2 | 170 | 156 | < 125 |
| 41 | H | 4-ClC6H4CH2 | 65 | 81 | < 125 |
| 42 | H | 4-BrC6H4CH2 | 90 | 108 | < 125 |
| 43 | H | 2-NaphthylCH2 | 56 | N.D. | N.D. |
| 56 | C2H5 | 4-ClC6H4CH2 | 6.3 | 6.2 | < 125 |
| 57 | n-C3H7 | 4-ClC6H4CH2 | 3.0 | 2.6 | < 125 |
| 58 | n-C6H13 | 4-ClC6H4CH2 | 2.0 | 1.8 | < 125 |
| 59 | n-C7H15 | 4-ClC6H4CH2 | 2.8 | 2.2 | < 125 |
| 60 | n-C8H17 | 4-ClC6H4CH2 | 16.0 | 12.0 | 1105 |
| 61 | 4-ClC6H4CH2 |
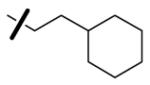
|
3.9 | 2.9 | 1007 |
| 62 | n-C6H13 | 4-FC6H4CH2 | 0.9 | 0.9 | 1462 |
| 63 | n-C8H17 | 4-FC6H4CH2 | 1.3 | 1.2 | < 125 |
| 64 | n-C6H13 | 4-BrC6H4CH2 | 2.9 | 2.8 | < 125 |
| 65 | n-C8H17 | 4-BrC6H4CH2 | 4.0 | 2.9 | < 125 |
| 66 | 4-ClC6H4CH2 | 4-ClC6H4CH2 | 6.1 | 4.8 | < 125 |
| 67 | 4-FC6H4CH2 | 4-FC6H4CH2 | 5.6 | 5.7 | < 125 |
| 68 | 4-BrC6H4CH2 | 4-BrC6H4CH2 | 14.0 | 11.0 | < 125 |
| 69 | 4-FC6H4CH2 | 4-ClC6H4CH2 | 6.1 | 6.1 | < 125 |
| 70 | 4-BrC6H4CH2 | 4-ClC6H4CH2 | 8.3 | 7.7 | < 125 |
| 71 | 4-BrC6H4CH2 | 4-FC6H4CH2 | 5.7 | 5.1 | < 125 |
| 72 | 2,4-Cl2C6H3CH2 | 2,4-Cl2C6H3CH2 | 12.6 | 11.0 | < 125 |
| 73 | 2,6-F2C6H3CH2 | 2,6-F2C6H3CH2 | 14.7 | 18.3 | < 125 |
| 74 | 3-FC6H4CH2 | 3-FC6H4CH2 | 5.1 | 6.7 | < 125 |
| 75 | 2-ClC6H4CH2 | 2-ClC6H4CH2 | 3.6 | 4.9 | < 125 |
N.D.: not determined
