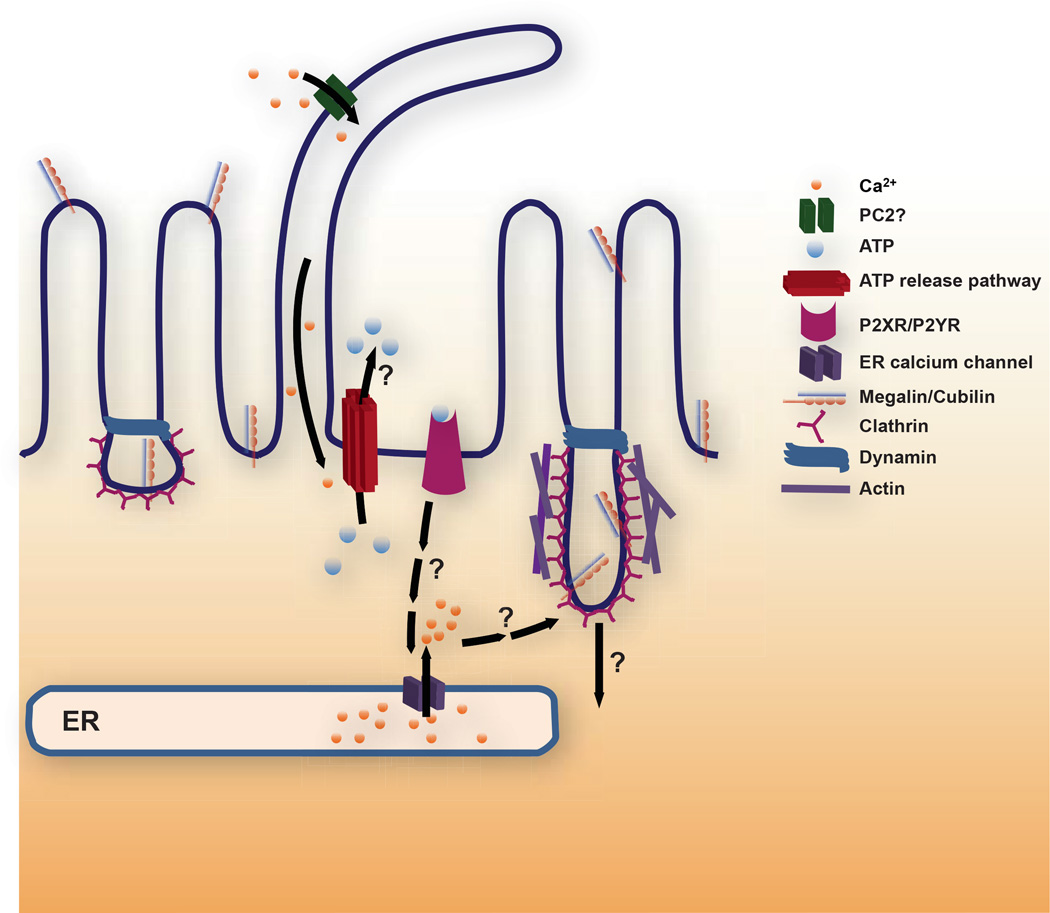
Figure 1. Working model for FSS-stimulated endocytosis in the proximal tubule.
We hypothesize that ciliary bending activates a cation channel [possibly polycystin-2 (PC2)] that elevates ciliary and periciliary [Ca2+]i to trigger luminal release of ATP via any of several pathways (see text for details). ATP activates purinergic P2X or P2Y receptors that ultimately leads to Ca2+ release from ER stores via ryanodine and/or IP3 receptor mediated pathways. Increased [Ca2+]i in turn ultimately leads to enhanced apical endocytosis. The downstream signaling cascade leading to the endocytic response remains to be determined. Endocytosis from the apical surface of polarized cells occurs at the base of microvilli via a pathway that requires clathrin and dynamin. Coated pit morphology at the apical surface of PT cells is highly irregular compared with other polarized epithelial cells consistent with the possibility that endocytosis is stimulated by an increase in individual endocytic pit size. Alternatively or in addition, the increase in endocytic capacity in response to FSS may be caused by an increase in the number of apical clathrin coated pits that form.