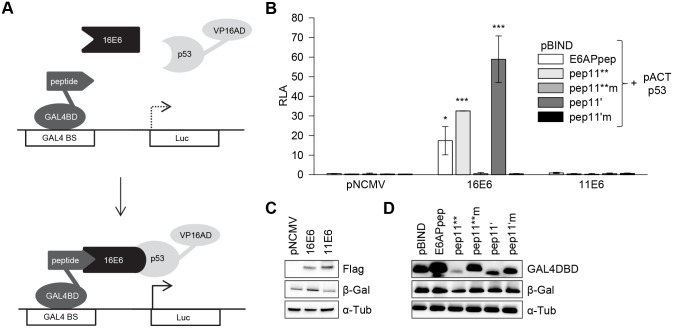
Fig 4. HPV16 E6 mediating intracellular formation of a trimeric complex with E6-binding peptides and p53.
(A) Schematic illustration of the employed modified mammalian two hybrid assay. Upper panel: Individual peptides are linked to GAL4-BD, p53 is linked to VP16-AD, E6 proteins are expressed from a co-transfected expression vector. Lower panel: Trimeric complex formation is detected by activation of the luciferase reporter. GAL4 BS, GAL4 binding sites. (B) Co-expression of individual peptides linked to the GAL4-BD, as indicated, together with p53-VP16-AD and HPV16 E6 or HPV 11 E6, respectively. Empty expression vector pNCMV served as negative control. Indicated are relative luciferase activities (RLA) above those of control-transfected cells, expressing the corresponding peptide-GAL4-BD fusions together with the co-transfected empty vectors pACT and pNCMV; values are arbitrarily set at 1.0. Results were obtained from three individual experiments, each performed in duplicates. Standard deviations are indicated. Asterisks above the columns indicate significant differences above those of cells expressing the corresponding peptides linked to GAL4-BD together with p53-VP16-AD, in the absence of E6 (co-transfected empty vector pNCMV), with p-values of ≤0.001 (***) and ≤0.05 (*). (C) Immunoblot analyses of expression levels of Flag-tagged HPV16 E6 and HPV11 E6 and of (D) individual peptides linked to GAL4-DB. Loading of protein extracts was normalized for equal transfection efficiencies, as determined by a co-transfected β-galactosidase expression vector. β-Gal, β-galactosidase; α-Tub, α-tubulin.