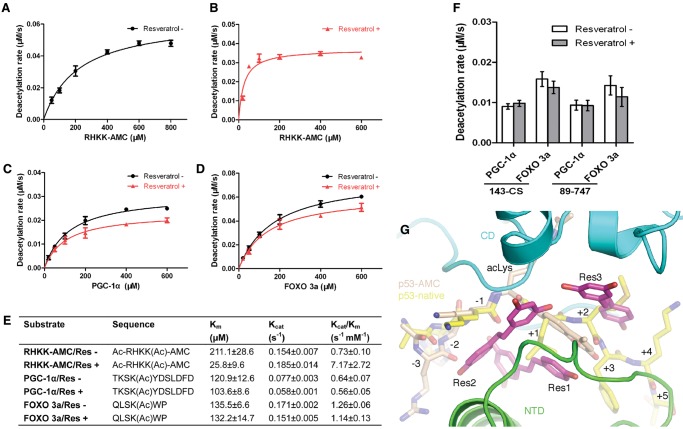
Figure 5.
Resveratrol effect on native peptides. (A–D), Titration curves for determination of Km values of SIRT1-143CS toward the indicated peptides in the absence (black curves) and presence (red curves) of 0.2 mM resveratrol. An enzyme concentration of 200 nM in B, as opposed to 400 nM in the rest, was used to slow down the reaction for better precision measurement. (E) A summary of measured Michaelis-Menten kinetic parameters for the indicated peptides in the absence and presence of 0.2 mM resveratrol. (F) Deacetylase activities of SIRT1 fragments 143-CS and 89–747 toward the indicated peptides measured at 400 nM and 50 μM enzyme and peptide concentrations, respectively, in the absence and presence of 0.2 mM resveratrol. (G) Superposition of the fluorophoreless acetylated p53 peptide (carbon colored yellow) from an archaeal sirtuin complex (PDB ID 1MA3) with the SIRT1 ternary complex. Residue positions with respect to the acetylated lysine are indicated.