Abstract
Introduction:
Arteriovenous fistula (AVF) is the gold standard vascular access for hemodialysis (HD). A thrill or murmur immediately after creation of AVF is considered a predictive sign of success. However, this does not ensure final maturation for successful HD. Our objective was to determine different clinical and duplex parameters within AVF to predict maturation and subsequent successful HD.
Materials and Methods:
A prospective observational study was conducted on 187 patients who had AVF formation from July 2012 to May 2013. Following surgery, all patients had Doppler ultrasound (DU) on Days 0 and 7. Doppler parameters noted in the outflow vein were: Thrill, broadening of spectral waveform with increased peak systolic velocity (PSV) and spiral laminar flow (SLF). Patients with at least one positive parameter at Day 0 were followed-up serially and underwent repeat Doppler imaging on Day 7. Patients with the absence of all three parameters on Day 0 were excluded from the study. Endpoint was maturation of AVF, i.e. successful HD. Statistical analysis was performed with binary logistic regression, to find out the strongest and earliest predictor for maturation of AVF using SPSS version 20.
Results:
SLF and broadening of spectral waveform with increased PSV were found to have a significant association with maturation (P = 0.0001). Presence of SLF on Day 0 most strongly predicted maturation. Presence of thrill or murmur could not predict the maturation.
Conclusions:
SLF pattern in AVF is the most important and the earliest predictor of maturation.
Keywords: Doppler, fistula, flow patterns, hemodialysis, predictor, spiral laminar
INTRODUCTION
Vascular access is the lifeline for a hemodialysis (HD) patient, but its creation and maintenance is a difficult undertaking. The arteriovenous fistula (AVF) has long been recognized as the preferred access.[1,2] Pre-operative evaluation of upper extremity veins and arteries with Doppler ultrasound is a useful adjunct to physical examination, especially for those patients who are obese, have had multiple previous access surgeries or otherwise are difficult to examine well, or for those in whom arterial or venous disease is suspected.[3,4,5] A palpable thrill or murmur after the creation of AVF is often attributed as a sign of immediate success of AVF, but this does not ensure the final maturation of AVF and subsequent successful HD. Even after creation of the AVF, close clinical and radiological, i.e. Doppler, surveillance is required to identify and localize abnormalities, which may potentially threaten access function and patency. Identification and correction of access abnormalities at early stages may decrease the waiting time to maturation and successful HD and improve longevity and function.[6,7] The review of the existing literature does not reveal any definite Doppler parameters in this regard. We therefore studied the different Doppler patterns of flow in the outflow vein along with other parameters to predict the maturation of AVF at 6 weeks. The aim of this study is to determine the earliest and strongest predictors of maturation of AVF so as to decrease the morbidity associated with a delay in starting successful HD.
MATERIALS AND METHODS
This prospective, observational study was conducted after Institutional Ethics Committee clearance. All patients of end-stage renal disease (ESRD), irrespective of age, admitted for AVF formation from July 2012 to May 2013, were included in the study after taking consent. Pre-operative Doppler was performed in selected patients. (This was based on the discretion of the operating surgeon, taking into account subjective assessment of upper limb veins and other factors like previous failed surgery, edematous upper limb and history of ipsilateral internal jugular vein cannulation or “permacath” insertion). Surgery was performed by the surgeons with experience of at least 1000 arteriovenous access procedures. Surgery was performed under local anesthesia, brachial block or general anesthesia as and when required. Type of AVFs made were radiocephalic (RCF), high radiocephalic (HRCF), brachiocephalic (BCF), brachio mediancubital (BMCF) or brachio basilic fistula with basilic vein transposition (BVT), depending on the history, clinical examination or Doppler findings. End (arterial) to side (venous) anastomosis was performed in all patients. Doppler examination (DU) was performed in all patients post-operatively on Day 0 and presence or absence of three parameters were noted in the outflow vein as thrill or murmur, spectral broadening and the spiral laminar flow (SLF). SLF was visible as red blue shift on Doppler. Presence of any of the above three parameters was the criteria used to follow the patient till Day 7. On post-operative Day 7, all three parameters were again assessed and the presence of any of the above three parameters was the criteria used to follow the patient till 6 weeks for maturation of AVF and successful hemodialysis (HD). The information regarding successful HD in a patient was acquired from the hospital information system at our dialysis unit or was collected telephonically in case the patients was getting HD at another place. Patients with absence of all three parameters on Day 0 were excluded from the study as it implied primary failure. Endpoint was defined as maturation of AVF, i.e. successful hemodialysis at 6 weeks.
Data of all patients were recorded in a selected format. Statistical analysis was performed with SPSS version 20. Statistical significance, i.e. P < 0.05, of different predictors for maturation of AVF was calculated by descriptive statistics using crosstabs via the Pearson chi square test. Then, by binary logistic regression analysis, regression coefficient B and exponent B was calculated for different predictors using 95% confidence intervals (CI) to determine the strongest and earliest predictors for maturation of AVF at 4–6 weeks.
RESULTS
The total number of patients was 203. A total 16 patients were excluded in view of absence of all three parameters on Day 0. The remaining 187 patients were included in the study. Age ranged from 7 to 85 years (mean age, 48.2 ± 15.38 years). One hundred and six patients were male and 81 patients were females. Forty-two patients had type 2 diabetes mellitus and 43 patients had hypertension. A total 121 RCF, six HRCF, 48 BCF, six BMCF and six BVT were made.
Parameters on Day 0
SLF was present in 161 (86.1%) patients. Of the 161 patients, 137 (85.1%) patients had successful HD at 6 weeks. AVF failed to mature in 24 (14.9%) patients at 6 weeks. SLF was absent in 26 (13.9%) patients. Of the 26 patients, only five (19.2%) patients had successful HD at 6 weeks. AVF failed to mature in 21 (80.8%) patients at 6 weeks [Table 1].
Table 1.
Parameter wise distribution of outcome at day 0
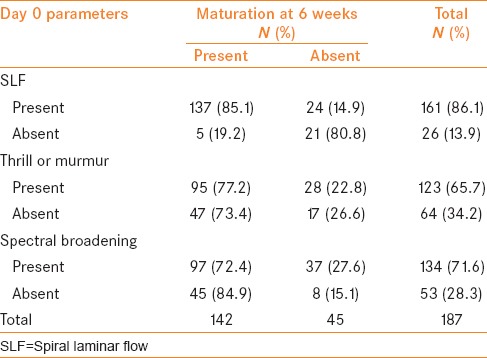
Thrill or murmur was present in 123 (65.7%) patients. Of the 123 patients, 95 (77.2%) patients had successful HD at 6 weeks. AVF failed to mature in 28 (22.8%) patients at 6 weeks. It was absent in 64 (34.2%) patients. Of the 64 patients, 47 (73.4%) patients had successful HD at 6 weeks. AVF failed to mature in 17 (26.6%) patients at 6 weeks [Table 1].
Spectral broadening was present in 134 (71.6%) patients. Of these 134 patients, 97 (72.4%) patients had successful HD at 6 weeks. AVF failed to mature in 37 (27.6%) patients at 6 weeks. It was absent in 53 (28.3%) patients. Of the 53 patients, 45 (84.9%) patients had successful HD at 6 weeks. AVF failed to mature in eight (15.1%) patients at 6 weeks [Table 1].
Parameters on Day 7
SLF was present in 138 (73.7%) patients. Of these patients, 128 (92.8%) patients had successful HD at 6 weeks. AVF failed to mature in 10 (7.2%) patients at 6 weeks. SLF was absent in 49 (26.3%) patients. Of these 49 patients, 14 (28.6%) patients had successful HD at 6 weeks. AVF failed to mature in 35 (18.7%) patients at 6 weeks [Table 2].
Table 2.
Parameter-wise distribution of outcome on Day 7
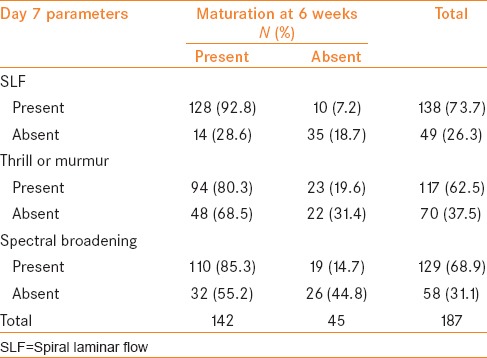
Thrill or murmur was present in 117 (62.5%) patients. Of the 117 patients, 94 (80.3%) patients had successful HD at 6 weeks. AVF failed to mature in 23 (19.6%) patients at 6 weeks. It was absent in 70 (37.5%) patients. Of these 70 patients, 48 (68.5%) patients had successful HD at 6 weeks. AVF failed to mature in 22 (31.4%) patients at 6 weeks [Table 2].
Spectral broadening was present in 129 (68.9%) patients. Of these 129 patients, 110 (85.3%) patients had successful HD at 6 weeks. AVF failed to mature in 19 (14.7%) patients at 6 weeks. This was absent in 58 (31.1%) patients. Of the 58 patients, 32 (55.2%) patients had successful HD at 6 weeks. AVF failed to mature in 26 (44.8%) patients at 6 weeks [Table 2].
Statistical analysis
Day 0: Presence of SLF in the outflow vein at Day 0 was significantly associated with final maturation of AVF at 6 weeks, P value = 0.0001. SLF at Day 0 most strongly predicted the maturation (regression coefficient B -2.514, exponent B 0.081). Presence of thrill or murmur in the outflow vein on Day 0 had no significant association with maturation of AVF at 6 weeks, P value = 0.564. There was no significant association between the presence of spectral broadening in the outflow vein on Day 0 with final maturation of AVF at 6 weeks, P value = 0.071. Among the three predictors, the positive and negative predictive values (PPV, NPV) on Day 0 were the highest for SLF (PPV - 85.09%, NPV - 80.70%) [Table 3].
Table 3.
Positive and negative predictive values of each parameter on Days 0 and 7
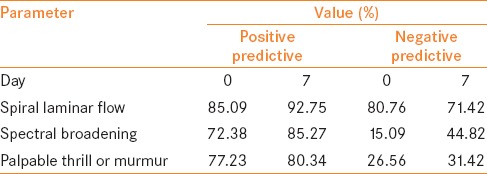
Day 7: Presence of SLF in the outflow vein on Day 7 was significantly associated with final maturation of AVF at 6 weeks, P value = 0.0001. No significant association was found between between thrill and murmur in the outflow vein on Day 7 with final maturation of AVF at 6 weeks, P value = 0.068. Spectral broadening in the outflow vein on Day 7 was significantly associated with maturation of AVF at 6 weeks, P value = 0.0001. Among the three predictors, the PPVs and NPVs on Day 7 were the highest for SLF (PPV - 92.75%, NPV - 71.42%) [Table 3].
In the presence of SLF, the presence or absence of co-morbidities did not affect the final maturation as, by applying the chi square test, the P values were 0.271 and 0.698, i.e. >0.05, at Days 0 and 7, respectively.
The presence of SLF on Day 0 was the strongest and earliest predictor of maturation of AVF at 6 weeks. Spectral broadening on Day 7 had a significant correlation but was not as strong a predictor of maturation of AVF as SLF. In this study, presence of thrill or murmur had no association with maturation of AVF.
DISCUSSION
Dysfunction of vascular access (VA) is the first cause of morbidity in end-stage renal disease patients undergoing HD. Maintenance of adequate flow in VA to ensure successful dialysis is a priority in dialysis units.[8] In HD, the gold standard and most reliable VA is an AVF.[1,2] AVF cannot be used immediately for HD after formation. It takes at least 4–6 weeks for maturation and to be used for successful HD. Unfortunately, there is a long list of patients unnecessarily waiting for maturation of the AVF even after 6 weeks, and 20–30% fail to mature finally. In most cases, the etiology of AVF failure largely remains undetermined.
Following the creation of AVF, the prediction for maturation has traditionally been based on the presence of palpable thrill or pan systolic murmur. In the era of Doppler ultrasound, the role of palpable thrill or murmur to predict maturation of AVF is debatable. Regular examination of newly formed AVF with Doppler helps to detect factors that could lead to the failure of AVF. The combination of these modalities provided both anatomical and hemodynamic analysis of the VA in patients whose AVF is not yet used for dialysis delivery. The review of the existing literature did not reveal any definitive predictors for the maturation of the fistula, which could lead to successful HD.
We studied some new parameters such as presence of SLF or broadening of spectral waveform in order to determine the factors that could predict the chances of maturation of AVF at the earliest.
Normal physiological blood flow is laminar, i.e. non-turbulent. After leaving the left ventricle of the heart, blood reaches the aortic arch in a distinctive single spiral flow pattern.[9,10] In 1991, Stonebridge and Brophy presented their work on the three-dimensional nature of blood flow and postulated that the normal physiological blood flow pattern was SLF (rotating or helical laminar flow) [Figure 1a and b].[11]
Figure 1.
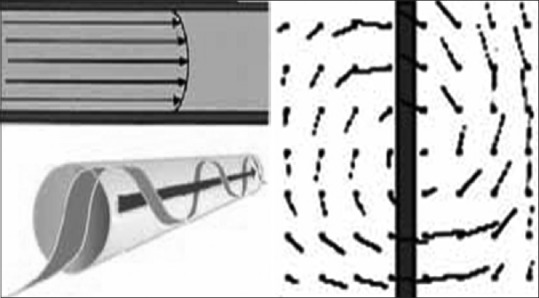
Three-dimensional blood flow pattern in a vessel
There are a number of beneficial hemorrheological properties associated with SLF.[12] It has laminar stability, reduced laterally directed forces and near-wall turbulence. In SLF, there is no increase in platelet activation; therefore, it suppresses acute thrombus formation. It enhances oxygen flux to the arterial wall, reduces luminal surface low-density lipoprotein concentration, dampens wall stress temporal gradients and lowers oscillatory shear stress index and thereby reduces the chances of neointimal hyperplasia.
The spiral flow pattern is readily identified using Doppler by interrogating blood vessels in a true transverse plane at low-velocity settings. This approach produces a characteristic “red/blue” split to the transverse color Doppler image [Figure 2a]. If SLF is disrupted because of physiological and anatomical reasons, turbulent blood flow results. There is a loss of red blue shift in the area of turbulence [Figure 2b]. Studies have shown that where SLF is lacking, arterial disease severity and progression is greater.[13]
Figure 2.
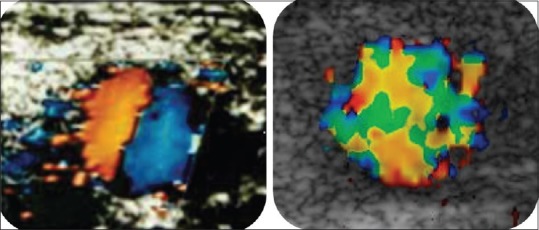
“Red blue” shift and turbulent blood flow on Doppler in transverse plane in the outflow vein
In cardiovascular surgery, the presence of SLF in cardiopulmonary bypass graft is considered as a positive predictor of graft patency in view of its above-said advantages. Many prosthetic grafts fail owing to the absence of SLF, as noted by Stonebridge et al.[11,12,13,14,15] Talbot in 2012 postulated that morphological changes in the spectral waveform suggested significant disease. This was associated with hemodynamic disturbance with loss of the normal triphasic waveform.[16] Concept of Doppler flow patterns in AVF is being applied to diagnose factors leading to non-maturation of AVF. KDOQI had issued clinical practice guidelines for vascular access, in which broadening of spectral waveform with increased peak systolic velocity (PSV) in the draining, i.e. outflow vein, [Figure 3] was taken as a sign of maturation of AVF.[1,2]
Figure 3.
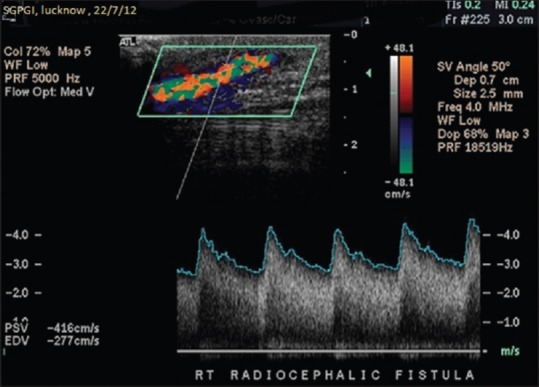
Normal arteriovenous fistula demonstrating marked spectral broadening and elevated peak systolic velocity
We applied the concept of three-dimensional flow patterns, SLF in the outflow vein that could predict the maturation of AVF [Figure 4]. In the present study, after satisfying the inclusion and exclusion criteria, Doppler examination was performed on Days 0 and 7. Presence or absence of three parameters as palpable thrill or pan systolic murmur, broadening of spectral waveform and SLF were noted and patients were followed till 6 weeks.
Figure 4.
Doppler of arteriovenous fistula demonstrating red blue shift (spiral laminar flow) in the outflow vein on Day 0
In this study, of all the three parameters on Day 0, presence of SLF strongly predicted the maturation of AVF at 6 weeks (P = 0.0001, exponent B 0.081 and regression coefficient (B) - 2.514). The presence of palpable thrill or pan systolic murmur on Day 0 (P = 0.564) and on Day 7 (P = 0.068) could not predict final maturation. Broadening of spectral waveform with increase in peak systolic velocity on Day 0 could not predict maturation at 6 weeks (P = 0.071). But, presence of the above finding along with SLF on Day 7 predicted maturation significantly (P = 0.0001). Even on Day 7, presence of SLF alone was the strongest predictor (P = 0.0001).
In view of the above findings, it was suggested that presence of SLF pattern on Doppler examination was the earliest predictor of final maturation at 6 weeks and subsequent successful HD.
The clinical significance of this study is that based on the presence or absence of SLF on Day 0 and 7 patients can be informed regarding the success or failure of maturation, i.e. possible outcome of AVF. Hence, decision regarding alternate access can be taken earlier and patients need not wait for the customary 4–6 weeks period, which could sometimes extend up to 3–6 months depending upon the variable practices in a particular center. This can decrease the unnecessary waiting time for successful HD and eventually decrease the morbidity.
CONCLUSIONS
SLF in the outflow vein denotes the propagation of natural blood flow in newly created AVF. Presence of SLF pattern on Doppler in the outflow vein of AVF as HD access can be considered as the most important factor in predicting the maturation of AVF at the earliest. A thrill or murmur cannot reliably predict maturation of AVF and subsequent successful HD.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.NKF-DOQI clinical practice guidelines for vascular access. National Kidney Foundation-Dialysis Outcomes Quality Initiative. Am J Kidney Dis. 1997;30(4 Suppl 3):150–91. [PubMed] [Google Scholar]
- 2.Vascular Access 2006 Work Group: Clinical practice guidelines for vascular access. Am J Kidney Dis. 2006;48(Suppl 1):176–247. doi: 10.1053/j.ajkd.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 3.Michael BS, Robert WH, 2nd, Peter JP, Zafar J, Clifford TA, Mark CG, et al. A strategy for increasing use of autogenous hemodialysis access procedures: Impact of preoperative non invasive evaluation. J Vasc Surg. 1998;27:302–8. doi: 10.1016/s0741-5214(98)70360-x. [DOI] [PubMed] [Google Scholar]
- 4.Ferring M, Henderson J, Wilmink A, Smith S. Vascular ultrasound for the pre-operative evaluation prior to arteriovenous fistula formation for haemodialysis: Review of the evidence. Nephrol Dial Transplant. 2008;23:1809–15. doi: 10.1093/ndt/gfn001. [DOI] [PubMed] [Google Scholar]
- 5.Ferring M, Claridge M, Smith SA, Wilmink T. Routine preoperative vascular ultrasound improves patency and use of arteriovenous fistulas for hemodialysis: A randomized trial. Clin J Am Soc Nephrol. 2010;5:2236–44. doi: 10.2215/CJN.02820310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kumbar L, Karim J, Besarab A. Surveillance and monitoring of dialysis access. Int J Nephrol. 2012;2012:649735. doi: 10.1155/2012/649735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paulson WD, Moist L, Lok CE. Vascular access surveillance: An ongoing controversy. Kidney Int. 2012;812:132–42. doi: 10.1038/ki.2011.337. [DOI] [PubMed] [Google Scholar]
- 8.Besarab A, Asif A, Roy-Chaudhury P, Spergel LM, Ravani P. The native arteriovenous fistula, surveillance and monitoring. J Nephrol. 2007;20:656–67. [PubMed] [Google Scholar]
- 9.Buckberg GD. Basic science review: The helix and the heart. J Thorac Cardiovasc Surg. 2002;124:863–83. doi: 10.1067/mtc.2002.122439. [DOI] [PubMed] [Google Scholar]
- 10.Marinelli R, Fuerst B, Zee H, McGinn A, Marinelli W. Philadelphia, Pa: Temple University; 1995. The heart is not a pump: A refutation of the pressure propulsion premise of heart function. Frontier Perspectives, J Centre Frontier Sciences; p. 5. [Google Scholar]
- 11.Stonebridge PA, Brophy CM. Spiral laminar flow in arteries. Lancet. 1991;338:1360–1. doi: 10.1016/0140-6736(91)92238-w. [DOI] [PubMed] [Google Scholar]
- 12.Stonebridge PA, Hoskins PR, Allan PL, Belch JF. Spiral laminar flow in vivo. Clin Sci (Lond) 1996;91:17–21. doi: 10.1042/cs0910017. [DOI] [PubMed] [Google Scholar]
- 13.Houston JG, Gandy SJ, Milne W, Dick JB, Belch JJ, Stonebridge PA. Spiral laminar flow in the abdominal aorta: A predictor of renal impairment deterioration in patients with renal artery stenosis? Nephrol Dial Transplant. 2004;19:1786–91. doi: 10.1093/ndt/gfh238. [DOI] [PubMed] [Google Scholar]
- 14.Houston JG, Gandy SJ, Sheppard DG, Dick JB, Belch JJ, Stonebridge PA. Two-dimensional flow quantitative MRI of aortic arch blood flow patterns: Effect of age, sex, and presence of carotid atheromatous disease on prevalence of spiral blood flow. J Magn Reson Imaging. 2003;18:169–74. doi: 10.1002/jmri.10341. [DOI] [PubMed] [Google Scholar]
- 15.Stonebridge PA. Three-dimensional blood flow Dynamics: Spiral/helical laminar Flow. Methodist Debakey Cardiovasc J. 2011;7:21–6. doi: 10.14797/mdcj-7-1-21. [DOI] [PubMed] [Google Scholar]
- 16.Talbot SR. Assessment of upper extremity arterial occlusive disease. In: Pellerito JS, Polak J, editors. Introduction to vascular ultrasonography. Philadelphia PA: Elsevier; 2012. pp. 262–80. [Google Scholar]


