Figure 1. Changes in dendritic cell metabolism through development, quiescence and activation.
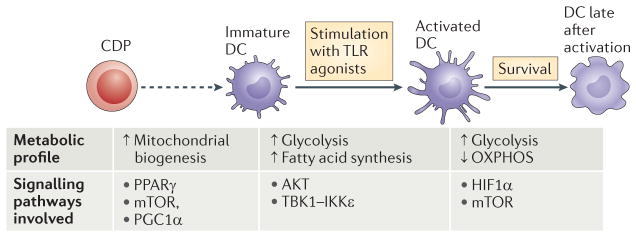
The development of dendritic cells (DCs) from progenitor cells is associated with mitochondrial biogenesis, which is driven by peroxisome proliferator-activated receptor-γ (PPARγ) co-activator 1α (PGC1α) and promoted by PPARγ, mammalian target of rapamycin (mTOR) and MYC. Differentiated DCs populate their niches as immature DCs. Immature DCs use fatty acid oxidation as a core metabolic process. Activation of DCs by Toll-like receptor (TLR) agonists leads to a rapid increase in flux through glycolysis and the associated pentose phosphate pathway, with an accompanying increase in spare respiratory capacity and fatty acid synthesis. These metabolic changes are initiated by a pathway downstream of TLRs that involves AKT, TANK-binding kinase 1 (TBK1), inhibitor of nuclear factor-κB kinase subunit-ε (IKKε) and hexokinase 2 (HK2), and they are crucial for DC activation. After being activated, DCs remain glycolytic. This process is essential for continued DC survival and is controlled by mTOR and hypoxia-inducible factor 1α (HIF1α). CDP, committed DC progenitor; OXPHOS, oxidative phosphorylation.
