Abstract
Cognitive operations often require integration of information. Previous studies have shown that integration of information in working memory recruits frontopolar cortex (FPC). In this fMRI study, we sought to reveal neural mechanisms of FPC underlying the integration of information during arithmetic tasks. We compared a condition requiring manipulation of two features of an item held in working memory with manipulation of one feature. The results showed that FPC was equally recruited in both conditions while dorsolateral prefrontal cortex (DLPFC) tended to be more activated when manipulating two features. We suggest that FPC plays an integrative role and is recruited by the production of representations in accordance with task constraints whereas DLPFC appears to be sensitive to processing demands induced by the manipulation of information.
Keywords: functional MRI, frontopolar cortex, dorsolateral prefrontal cortex, working memory, manipulation, integration
INTRODUCTION
Working memory (WM) plays a central role in complex cognitive activity, allowing us to temporarily maintain and manipulate task-related information. A number of studies have explained the neural mechanisms underlying the WM system [e.g., 6, 11, 13, 18]. These studies, however, have dealt with tasks using relatively simple stimuli (i.e., single dimensional stimuli) such as objects or spatial locations. In contrast, a relatively small number of studies have focused on how the brain integrates two or more unrelated items held in WM [e.g., 2, 8, 9, 14, 20]. According to Baddeley’s model [1], WM consists of the central executive, the visuospatial sketchpad, the phonological loop, and the episodic buffer. Baddeley proposed that the episodic buffer works in conjunction with the visuospatial and phonological stores, binding visuospatial and phonological information together into episodic WM representations. The episodic WM store acts as a buffer through which these representations migrate to long term episodic memory. However, the underlying neural mechanisms and substrates involved in binding the visuaospatial and phonological information to each other are integrated into single memory chunks are still unclear.
In this context, previous neuroimaging studies have sought to reveal the neural mechanisms of integration of information held in WM. Specifically, Prabhakaran et al. [20] demonstrated that holding integrated information during a WM task recruits frontopolar cortex (FPC). In their study, participants were asked to maintain four letters and four spatial locations indicated by parentheses. In one condition, each of the four letters appeared within one of the parentheses, such that each of them was bound into a single item. In another condition, the letters were situated centrally and the four locations were spread around the screen. Comparison of the two conditions showed that FPC was activated when identity and location were integrated but not when they were encoded separately. Subsequently, they suggested that maintenance of integrated information recruits FPC.
De Pisapia et al. [9] found FPC involvement in integration using different tasks. In their mental arithmetic tasks requiring participants to integrate a preloaded digit (e.g., “6”) into an ongoing calculation when cued (e.g., “+_”), they observed FPC activation during integration. In a follow-up study, they replicated this [8]. However, the tasks used in these studies did not include the maintenance of multimodally-integrated information as in Prabhakaran et al. [20], but rather it required the integration process, which is an actively operative component. In line with these studies, Ramnani and Owen [21] reviewed different perspectives on FPC function including episodic memory retrieval [22], prospective memory [3], cognitive branching [16] and relational integration [14, 17] and subsequently suggested that the role of FPC is to integrate the products of two or more cognitive operations.
However, in the previous studies, FPC activation during WM tasks might have resulted from the complexity of executing the task since manipulation required participants to change two features of two items in WM. In a study that employed the Tower of London task to measure planning ability, for example, Van Den Heuvel et al. [24] found that FPC activity positively correlates with the number of moves required to transform the current state to the goal state. In other studies using Raven’s Progressive Matrices, FPC was recruited in a high complexity condition to a greater degree than it was in a low complexity condition [4, 17], suggesting that it responded to the demand to integrate the multiple relations.
However, the nature of FPC involvement observed in previous studies is still unclear. It’s not possible to differentiate, based on their findings, whether FPC is involved in manipulation of items held in WM or integration of them. The purpose of this study was to examine the neural mechanisms underlying integration-related processing. We tested whether FPC activation, which responds during manipulation of integrated information held in WM, is consistent with the hypothesis that FPC plays a role in production of integrated representations but not in executing manipulations of them. We designed a task consisting of a complex manipulation condition (CM) requiring participants to manipulate two features of an integrated item, a simple manipulation condition (SM) requiring them to manipulate only one feature of an item, and a control condition without integrated information (see Figure 1). We expected different activation patterns between FPC and DLPFC. Specifically, both CM and SM would recruit FPC with the same intensity but with a temporal disparity, because there is a single integrated representation to be formed within the episodic buffer in both conditions but there are different numbers of features to be integrated into the representation. For DLPFC, in contrast, we expected that activity would be greater in CM than in SM since the cognitive demands of manipulating information are greater in CM.
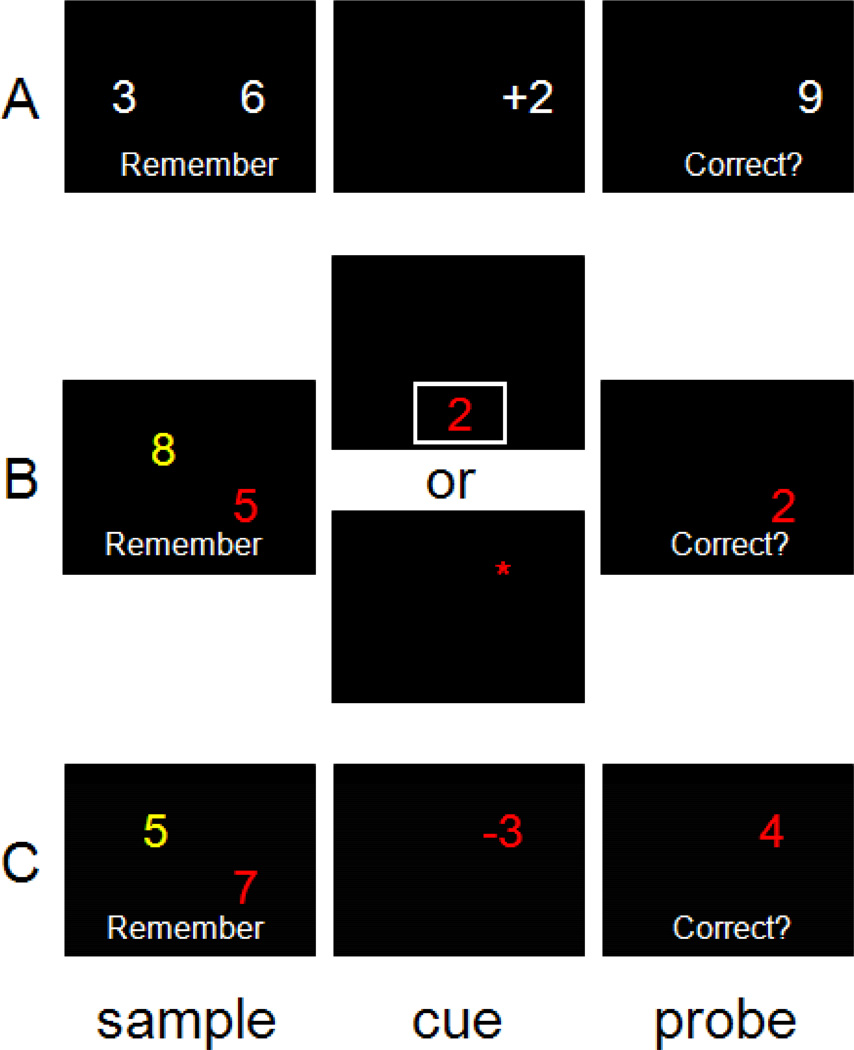
Figure 1.
Stimuli of each condition. A: Control condition. “6” should be added by “2”, resulting in “8”, and thus the correct answer is “incorrect”; B: Simple manipulation condition (SM). The cue is printed in red. Thus “5” in the sample should be changed to “2” or moved to the location cued by the asterisk; C: Complex manipulation condition (CM). Since the cue is printed in red, “7” in the sample should be subtracted by “3”, resulting in “4”, and moved to the location where the cue is presented. Thus the correct answer is “correct”.
METHODS
Participants
Twelve healthy right-handed, native English speakers (four males) with an age range of 19–31 (M=22.9, SD=3.9) participated in this study. Informed consent forms approved by the University of New Mexico Institutional Review Board were obtained from all participants.
Stimuli and procedure
Three different conditions were employed: the control condition, SM and CM (Figure 1). All trials began with two single digit numbers (the sample stimulus). A cue was then presented, followed by a probe stimulus. All stimuli were presented on a black screen. In the control condition, participants were asked to remember two numbers presented on the left and the right on a screen (e.g., “3” on the left and “6” on the right). Then an additive or a subtractive operator with a single digit was presented randomly on either the left or right side and participants internally calculated the resulting arithmetic problem (e.g., “+2” on the right; compute “6+2”). This was followed by a probe containing a number presented on the left or right. Participants were asked to indicate whether the number matched the result of the calculation (e.g., “9” on the right; match result?) or the same as the unused sample number (e.g., “3” on the left; match the left sample number?). Numbers in samples and cues ranged from 2 to 9, and in probes were less than 16.
In the SM and CM, participants were to remember two numbers presented in different colors (red, green, blue, yellow, and magenta) and positions (two locations among 20 potential predefined positions). The cue indicated one of the sample stimuli to be manipulated using a colored asterisk or number (SM) or a colored operator with number (CM). In the SM, participants saw a cue (asterisk or number) whose color matched one of the numbers in the sample. Note that the number cue for the SM was presented in a white rectangle in order to present the cue without spatial information. Then they were required to change the position of the matching sample number presented in the preceding sample phase to the position of the asterisk, or the identity of the matching sample number to the identity indicated by the number. For the CM, participants were asked to both calculate the arithmetic problem indicated by the cue (i.e., a colored operator and number) and change the number’s position into the new position indicated by the cue’s position. The probe presented a colored number in a position, to which participants were asked to indicate whether the number matched the result of the manipulation or the unused sample number in terms of its position or identity, which was cued by the color. Thus, only one of the two possible changes was required in the SM, while both were required in the CM. Once the change to their WM representation was made, the required response could be calculated using the new, changed information. Participants were asked to press either left (for “correct”) or right (for “incorrect”) button using their left or right thumbs. The response time was measured by taking time immediately after the probe screen was presented. All stimuli were presented for 1.5 sec with inter-stimulus-intervals (ISIs) varied from 1.5 to 4.5 secs (average 2.5 secs). Each type of trial for control, SM, and CM, was repeated 28 times and all trials were presented in a randomized order in each run. There were 14 trials for each condition in each run, resulting in a total of 84 trials in two runs.
Image acquisition
Whole brain images were acquired with a 3-Tesla Siemens Trio scanner at the Mind Research Network in Albuquerque, New Mexico. High-resolution anatomical images (256×256×192) were acquired using a T1-weighted MPRAGE imaging sequence. Functional images were acquired using a T2*-weighted echo-planar imaging (EPI) sequence, which comprised of 33 interleaved 3mm-skip-1mm slices (TR=2,000 ms, TE=29 ms, Flip=75°, FOV=240 mm, Matrix=64×64).
Data analyses
Accuracy and response time were analyzed using analyses of variance. Functional imaging data were analyzed with SPM5. After discarding the first 8 volumes for each run, functional images were corrected for temporal disparities in the slice timing by the sinc interpolation [15]. The corrected images were realigned to the first slice to correct spatial differences. The resampled images were coregistered with each subject’s MPRAGE image. The images were normalized to the MNI-152 image and smoothed with an 8-mm FWHM Gaussian kernel.
In the first level model for each subject, all event types and the motion parameter were included in a general linear model using a canonical hemodynamic response function (HRF). Contrast images, which revealed activity greater than in the implicit base line, were constructed for experimental events (the manipulation cues of the control, SM and CM conditions). These images were then analyzed at the second level using paired-sample t-tests and a threshold was applied at p<0.05 corrected for multiple comparisons using a method based on the false discovery rate [12]. Event-related BOLD signal changes were extracted from regions of interest (ROIs) which were defined as spheres with 4 mm radius within FPC and DLPFC activated by the comparison of CM versus control. These ROIs were then used in the confirmatory analyses for direct comparisons between SM and CM in FPC and DLPFC.
RESULTS
Behavioral data
We compared the accuracy and response time of the three different conditions. The accuracy of the conditions was significantly different in the context of the omnibus test, F(2,22)=6.644, mean squared error (MSE)=0.006, p=0.006 (η2 =0.377). In order to explore this result in detail, the orthogonal contrasts were conducted. The results showed that accuracy for SM (M=0.83, SD=0.10) and CM (M=0.81, SD=0.07) were significantly lower than for the control condition (M=0.92, SD=0.05), F(1,11)=11.325, MSE=0.009 p=0.006(η2 =0.507), whereas there was no difference between the two manipulation conditions, F(1,11)=0.619, MSE=0.01, p=0.448 (η2 =0.053). Mean response times for SM (M=1,174 ms, SD=170), CM (M=1,197 ms, SD=197), and control condition (M=1,132 ms, SD=153) were not significantly different, F(2,22)=1.897, MSE=6766.262, p=0.174 (η2 =0.147).
Imaging data
In order to identify and determine the functional ROIs in FPC and DLPFC, CM was compared to the control condition. Frontal areas showing significantly greater responses to CM, where integrated information was repositioned and simultaneously computed, than the control condition are listed in Table 1. These included bilateral inferior frontal gyrus (BA 9/47) and right middle frontal gyrus (BA 10/46).
Table 1.
Frontal areas significantly activated by CM vs. control condition and SM vs. control condition.
| Anatomical regions | side | BA | Coordinates (mm) | z | mm3 | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| CM vs. control condition | |||||||
| Inferior Frontal Gyrus | L | 9 | −33 | 10 | 27 | 3.53 | 2,700 |
| R | 47 | 39 | 32 | −4 | 4.05 | 621 | |
| Middle Frontal Gyrus | R | 46 | 42 | 22 | 24 | 4.25 | 6,426 |
| R | 10 | 30 | 52 | 0 | 4.15 | 1,080 | |
| SM vs. control condition | |||||||
| Middle Frontal Gyrus | L | 6 | −42 | 11 | 46 | 3.65 | 324 |
| R | 6 | 33 | 11 | 52 | 3.87 | 2,349 | |
| R | 10 | 30 | 53 | 3 | 3.86 | 945 | |
| R | 9 | 50 | 19 | 35 | 3.71 | 297 | |
Among these clusters, functional ROIs for FPC and DLPFC were defined by spheres showing peak activations of the two clusters in the right middle frontal gyrus. Additionally, frontal regions responding to SM were identified (see Table 1), including middle frontal gyrus (bilateral BA 6, right BA 9 and right BA 10). Note that additional regions activated by these contrast were not listed in Table 1.
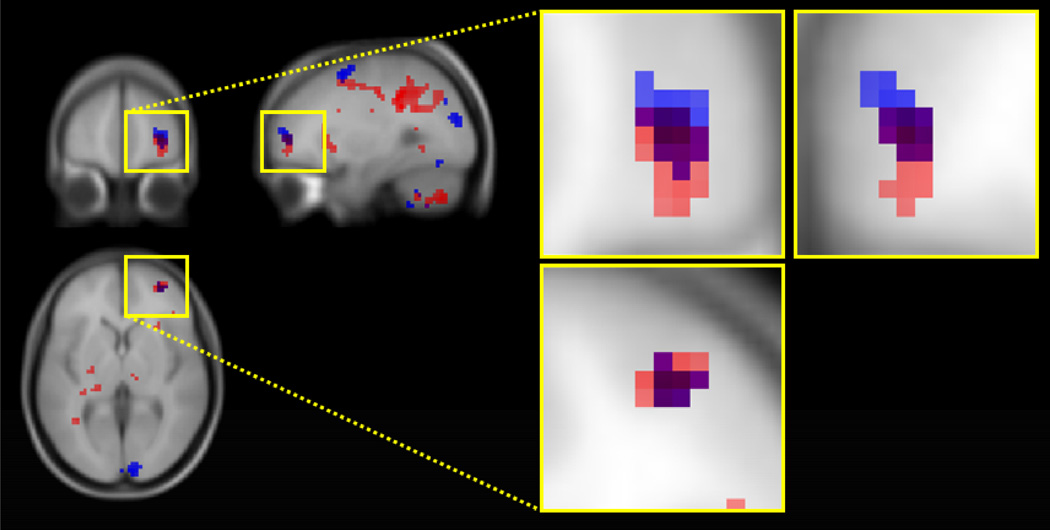
As shown in Figure 2 and Table 1, right FPC was activated for both SM and CM more than for the control. Most of the activations in FPC overlapped, but the area sensitive to SM was localized in a more dorsal area than the area involved in CM. On the other hand, far more voxels of right DLPFC (BA 9/46) were activated in CM (6426 mm3) than SM (297 mm3). Further, left DLPFC was activated only in contrasting CM with the control condition, but not SM with the control condition.
Figure 2.
Frontopolar activation during SM (blue) and CM (red) conditions relative to the control condition. The intersection of regions active in both comparisons is shown in violet. The maxima of the intersection area in frontopolar cortex seen in the sagittal view have a z-coordinate of 3 mm.
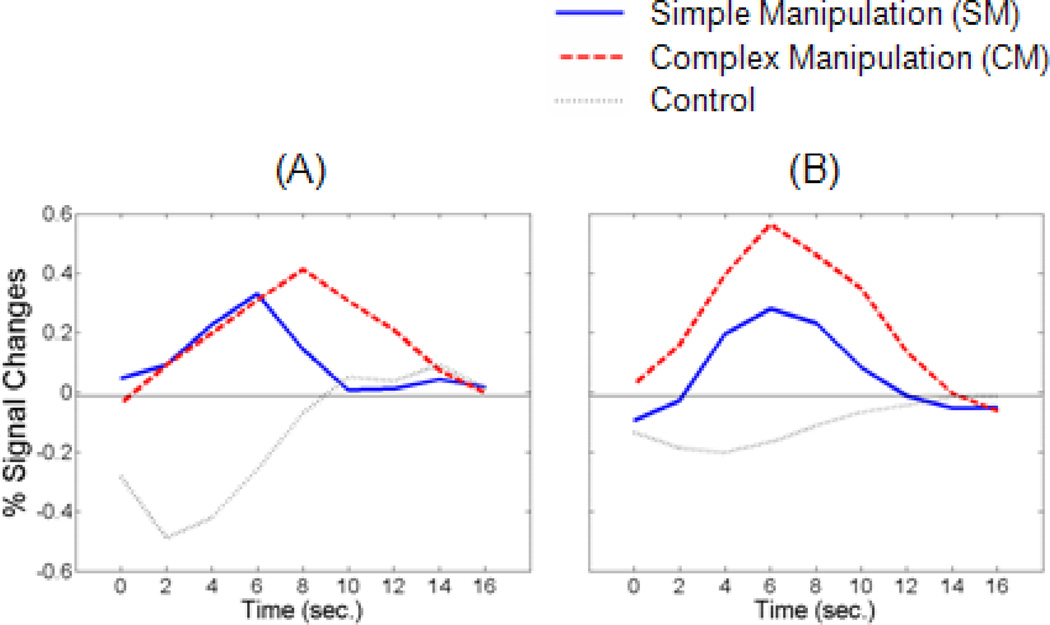
Direct comparisons of CM with SM within ROIs from FPC and DLPFC were conducted. Examination of the time courses of BOLD responses showed a different pattern of activation for these two conditions in FPC and DLPFC (Figure 3). Specifically, the peak amplitude of the signal changes for the ROI in FPC appeared to be higher in CM (M=0.41 %, SD=0.83) than in SM (M=0.33 %, SD=0.69), but they were not significantly different, t(11)= 0.233, p=0.820. However, the time-to-peak was more delayed in CM (M=8.33 sec, SD=2.39) than in SM (M=6.17 sec, SD=2.76) and these were significantly different, t(11)= 3.223, p=0.008. On the other hand, the time courses in DLPFC were more sensitive to manipulation demands; there was a stronger response to CM (M=0.56 %, SD=0.62) than to SM (M=0.28 %, SD=0.41), t(11)=2.505, p=0.029. However, the time-to-peak analysis showed no difference between CM (M=5.83 sec, SD=2.62) and SM (M=6.17 sec, SD=2.89), t(11)=0.272, p=0.791. Finally, we performed confirmatory ROI analyses to focus on activities in FPC and DLPFC to test whether these activations within the given ROIs were significantly different. The results showed that CM and SM were not significantly different in FPC (z=2.62, p=0.176, FDR-corrected), but the activation in DLPFC was significantly stronger in CM than SM (z=3.35, p=0.019, FDR-corrected).
Figure 3.
BOLD signal changes for ROIs in right FPC (BA 10; plot a) and right DLPFC (BA 46; plot b). The Talairach coordinates of the maxima of these two ROIs are 30,52,0 and 42,22,24.
DISCUSSION
The aim of the present study was to determine the roles of FPC and DLPFC during manipulation of integrated information. The results showed that FPC is involved in manipulation of integrated information held in WM even though the task required participants to manipulate only one feature of the integrated information. That is, the complexity of the manipulation did not affect the level of FPC activity. In contrast, DLPFC appeared to be more sensitive to the task complexity; the activity tended to show greater when two features were manipulated than when one was manipulated.
The time-to-peak of BOLD signal changes in FPC was delayed when participants manipulated two features relative to when they manipulated one. This was likely caused by production of a new representation for each manipulation, in which CM required more time than SM. Support for this explanation, a recent WM study for simple or complex stimuli (color or face, respectively) reported that encoding complex stimuli required longer times to successfully maintain representations than simple stimuli did. In accord with their behavioral results, lateral PFC also showed delayed time-to-peak for complex stimuli than simple stimuli [23]. Similarly, another study showed that PFC’s time-to-peaks increased linearly as a function of the number of items encoded in WM [10]. Thus, a reasonable interpretation of our results would be that delayed brain responses for CM relative to SM were associated with time to produce new integrated representations.
An alternative expression of these results is that FPC plays a role in binding, integrating, or directing the task processing in DLPFC, or in fact its management of the task across cortex. In this view, CM should recruit more of DLPFC and more intensely, because it requires a greater degree and amount of processing than SM. However, our findings support the idea that FPC is specialized for the production of integrated representations, and provides an alternate explanation for FPC activation in other studies [5, 6].
It might be argued that our findings are inconsistent with previous studies of manipulation of WM contents, in which manipulation of information held in WM depended on DLPFC [5, 7, 19]. However, these studies used relatively simple stimuli (e.g., a set of letters) and thus there was no demand for integration in their tasks or manipulation of integrated representations.
The present results suggest an explanation of FPC involvement in complex cognitive tasks, such as planning and reasoning. In the Raven's Progressive Matrices, Tower of London, and other high level tasks, representations must be manipulated into new representations in ways constrained by the inter-relationships. For example, in the Tower of London task requiring complex sequences of movements, one must produce a representation of possible move sequences based on a specific rule, in which the representation is constrained by the rule. Thus, the more the number of movements in the task, the greater FPC was activated [24]. Further, in the Raven’s Progressive Matrices task, complex problems have more changes or relations across the matrices, in which participants must deduce a missing cell in the matrix by the changes or relations among the other cells. This implies that complex matrices involve more constraints from the relationships within the matrix than simpler matrices [4, 17]. As these studies also showed, the corresponding increase in amount of manipulation required resulted in greater DLPFC recruitment.
In short, we suggest that FPC is recruited by production of highly constrained representations required during manipulation of integrative information held in WM. FPC activity did not increase with manipulation difficulty. We conclude that FPC is recruited when constrained representations are produced whereas DLPFC appears to be more sensitive to manipulation processing demands. This conclusion offers an explanation of neural mechanisms underlying FPC and DLPFC recruitment during high-level cognitive activities such as planning and reasoning.
Highlights.
-
➢
Frontopolar cortex was involved in integrating representations in working memory.
-
➢
Task complexity did not affect the level of the frontopolar cortex activation.
-
➢
In contrast, dorsolateral prefrontal cortex was sensitive to the task complexity.
ACKNOWLEGEMENTS
This work was supported by NIH grant S06 GM008136 and Los Alamos grant MOU-04-51.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Baddeley A. The episodic buffer: a new component of working memory? Trends Cogn Sci. 2000;4:417–423. doi: 10.1016/s1364-6613(00)01538-2. [DOI] [PubMed] [Google Scholar]
- 2.Badre D, Wagner AD. Selection, integration, and conflict monitoring; assessing the nature and generality of prefrontal cognitive control mechanisms. Neuron. 2004;41:473–487. doi: 10.1016/s0896-6273(03)00851-1. [DOI] [PubMed] [Google Scholar]
- 3.Burgess PW, Quayle A, Frith CD. Brain regions involved in prospective memory as determined by positron emission tomography. Neuropsychologia. 2001;39:545–555. doi: 10.1016/s0028-3932(00)00149-4. [DOI] [PubMed] [Google Scholar]
- 4.Christoff K, Prabhakaran V, Dorfman J, Zhao Z, Kroger JK, Holyoak KJ, Gabrieli JD. Rostrolateral prefrontal cortex involvement in relational integration during reasoning. Neuroimage. 2001;14:1136–1149. doi: 10.1006/nimg.2001.0922. [DOI] [PubMed] [Google Scholar]
- 5.Cohen JD, Perlstein WM, Braver TS, Nystrom LE, Noll DC, Jonides J, Smith EE. Temporal dynamics of brain activation during a working memory task. Nature. 1997;386:604–608. doi: 10.1038/386604a0. [DOI] [PubMed] [Google Scholar]
- 6.Curtis CE, D’Esposito M. Functional neuroimaging of working memory. In: Cabeza R, Kingstone A, editors. Handbook of Functional Neuroimaging of Cognition. London: MIT Press; 2006. pp. 269–306. [Google Scholar]
- 7.D'Esposito M, Postle BR, Ballard D, Lease J. Maintenance versus manipulation of information held in working memory: an event-related fMRI study. Brain Cogn. 1999;41:66–86. doi: 10.1006/brcg.1999.1096. [DOI] [PubMed] [Google Scholar]
- 8.De Pisapia N, Braver TS. Preparation for integration: the role of anterior prefrontal cortex in working memory. Neuroreport. 2008;19:15–19. doi: 10.1097/WNR.0b013e3282f31530. [DOI] [PubMed] [Google Scholar]
- 9.De Pisapia N, Slomski JA, Braver TS. Functional specializations in lateral prefrontal cortex associated with the integration and segregation of information in working memory. Cereb Cortex. 2007;17:993–1006. doi: 10.1093/cercor/bhl010. [DOI] [PubMed] [Google Scholar]
- 10.Druzgal TJ, D'Esposito M. Dissecting contributions of prefrontal cortex and fusiform face area to face working memory. J Cogn Neurosci. 2003;15:771–784. doi: 10.1162/089892903322370708. [DOI] [PubMed] [Google Scholar]
- 11.Fuster J. The Prefrontal Cortex. Philadelphia: Lippincott-Raven; 1997. [Google Scholar]
- 12.Genovese CR, Lazar NA, Nichols T. Thresholding of Statistical Maps in Functional Neuroimaging Using the False Discovery Rate. Neuroimage. 2002;15:870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- 13.Goldman-Rakic PS. Circuitry of primate prefrontal cortex and the regulation of behavior by representational memory. In: Plum F, editor. Handbook of Physiology. Vol. 5. Bethesda, MD: American Physiological Society; 1987. pp. 373–417. [Google Scholar]
- 14.Green AE, Kraemer DJM, Fugelsang JA, Gray JR, Dunbar KN. Connecting Long Distance: Semantic Distance in Analogical Reasoning Modulates Frontopolar Cortex Activity. Cerebral Cortex. 2010;20:70–76. doi: 10.1093/cercor/bhp081. [DOI] [PubMed] [Google Scholar]
- 15.Henson RN, Buchel C, Josephs O, Friston KJ. The slice-timing problem in event-related fMRI. Neuroimage. 1999;9:125. doi: 10.1006/nimg.1999.0498. [DOI] [PubMed] [Google Scholar]
- 16.Koechlin E, Basso G, Pietrini P, Panzer S, Grafman J. The role of the anterior prefrontal cortex in human cognition. Nature. 1999;399:148–151. doi: 10.1038/20178. [DOI] [PubMed] [Google Scholar]
- 17.Kroger JK, Sabb FW, Fales CL, Bookheimer SY, Cohen MS, Holyoak KJ. Recruitment of anterior dorsolateral prefrontal cortex in human reasoning: a parametric study of relational complexity. Cereb Cortex. 2002;12:477–485. doi: 10.1093/cercor/12.5.477. [DOI] [PubMed] [Google Scholar]
- 18.Petrides M, Alivisatos B, Meyer E, Evans AC. Functional activation of the human frontal cortex during the performance of verbal working memory tasks. Proc Natl Acad Sci U S A. 1993;90:878–882. doi: 10.1073/pnas.90.3.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Postle BR, D'Esposito M. Evaluating models of the topographical organization of working memory function in frontal cortex with event-related fMRI. Psychobiology. 2000;28:132–145. [Google Scholar]
- 20.Prabhakaran V, Narayanan K, Zhao Z, Gabrieli JD. Integration of diverse information in working memory within the frontal lobe. Nat Neurosci. 2000;3:85–90. doi: 10.1038/71156. [DOI] [PubMed] [Google Scholar]
- 21.Ramnani N, Owen AM. Anterior prefrontal cortex: insights into function from anatomy and neuroimaging. Nat Rev Neurosci. 2004;5:184–194. doi: 10.1038/nrn1343. [DOI] [PubMed] [Google Scholar]
- 22.Ranganath C, Johnson MK, D'Esposito M. Left anterior prefrontal activation increases with demands to recall specific perceptual information. J Neurosci. 2000;20:RC108. doi: 10.1523/JNEUROSCI.20-22-j0005.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Todd JJ, Han SW, Harrison S, Marois R. The neural correlates of visual working memory encoding: a time-resolved fMRI study. Neuropsychologia. 2011;49:1527–1536. doi: 10.1016/j.neuropsychologia.2011.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van den Heuvel OA, Groenewegen HJ, Barkhof F, Lazeron RH, van Dyck R, Veltman DJ. Frontostriatal system in planning complexity: a parametric functional magnetic resonance version of Tower of London task. Neuroimage. 2003;18:367–374. doi: 10.1016/s1053-8119(02)00010-1. [DOI] [PubMed] [Google Scholar]