Abstract
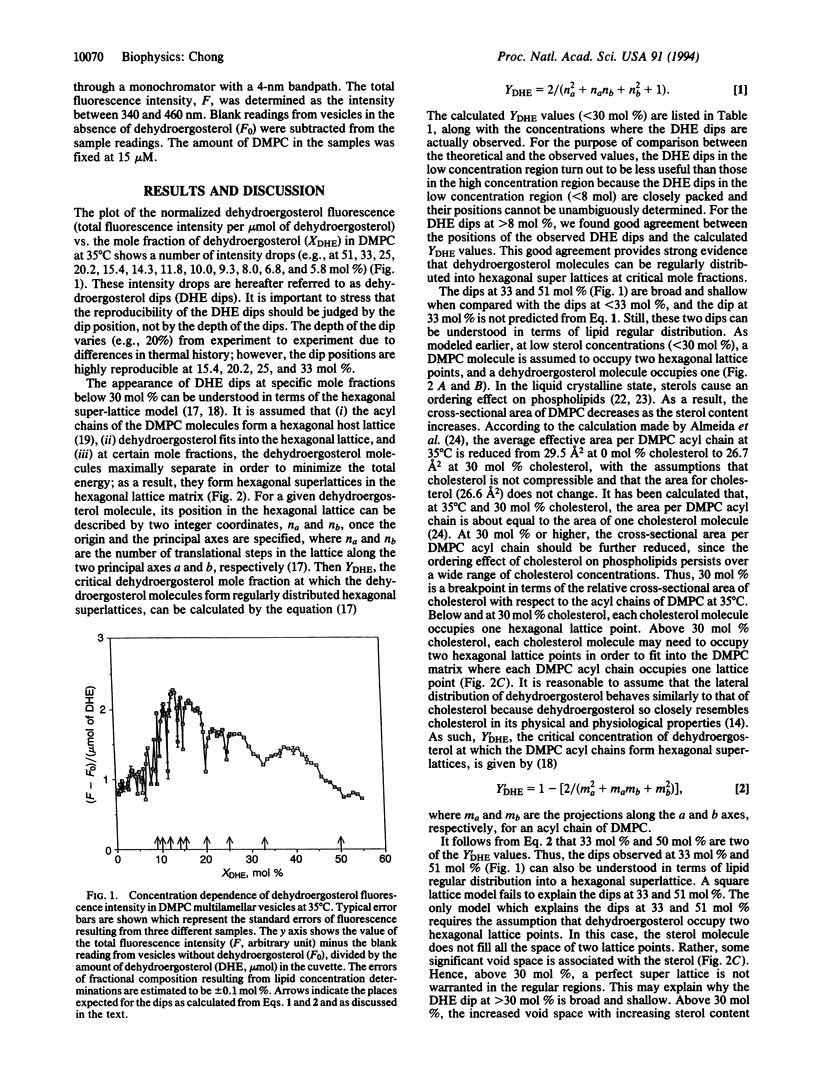
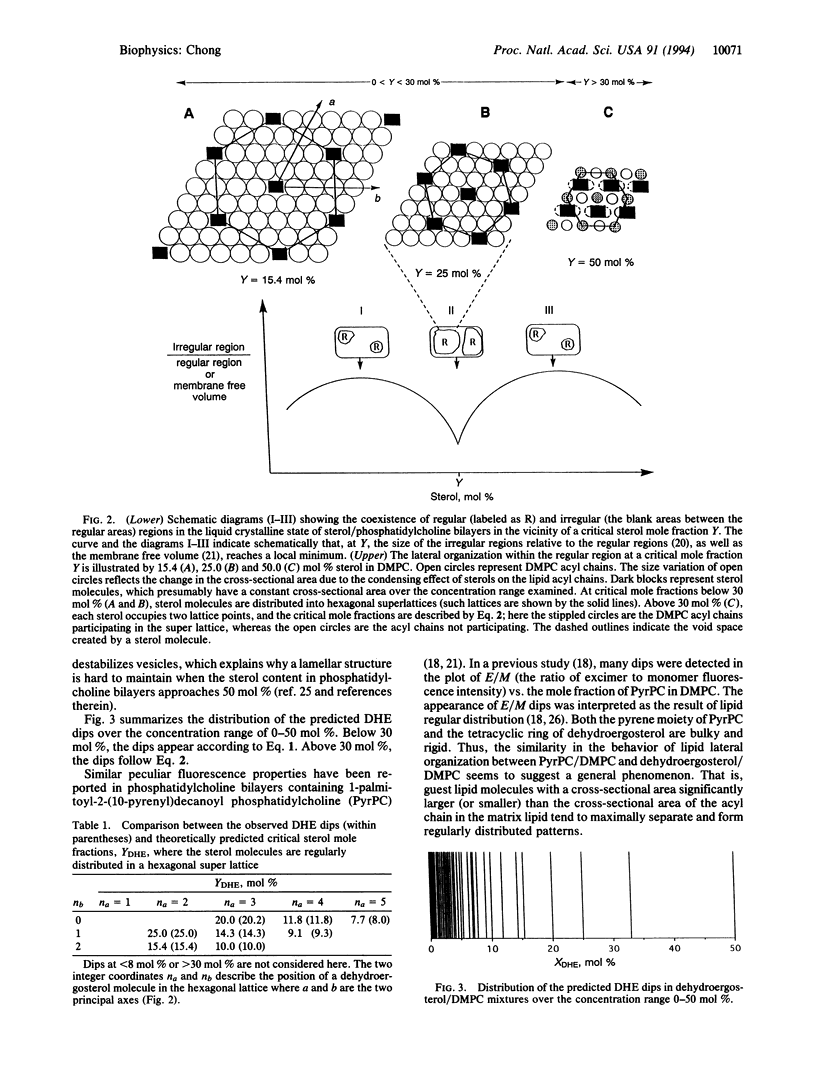
To investigate the lateral organization of sterols in membranes, the fluorescence intensity of dehydroergosterol at different mole fractions in liquid crystalline dimyristoyl phosphatidylcholine bilayers was examined. A number of intensity drops were observed at specific mole fractions, as predicted from a hexagonal super-lattice model. The fluorescence dips provide compelling evidence that a naturally occurring sterol is regularly distributed at fixed compositional fractions, consistent with the presence of hexagonal super-lattices in the fluid membranes. Regularly distributed regions, however, coexist with irregularly distributed regions. The extent of regular distribution varies periodically with sterol mole fraction and, consequently, similar variations take place in the membrane volume and lipid packing. This level of modulation in local membrane structure by minute changes in sterol concentration should have profound implications for the functional role of cholesterol content in cell membranes.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almeida P. F., Vaz W. L., Thompson T. E. Lateral diffusion in the liquid phases of dimyristoylphosphatidylcholine/cholesterol lipid bilayers: a free volume analysis. Biochemistry. 1992 Jul 28;31(29):6739–6747. doi: 10.1021/bi00144a013. [DOI] [PubMed] [Google Scholar]
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- Butko P., Hapala I., Nemecz G., Schroeder F. Sterol domains in phospholipid membranes: dehydroergosterol polarization measures molecular sterol transfer. J Biochem Biophys Methods. 1992 Mar;24(1-2):15–37. doi: 10.1016/0165-022x(92)90043-a. [DOI] [PubMed] [Google Scholar]
- Chapman D., Penkett S. A. Nuclear magnetic resonance spectroscopic studies of the interaction of phospholipids with cholesterol. Nature. 1966 Sep 17;211(5055):1304–1305. doi: 10.1038/2111304a0. [DOI] [PubMed] [Google Scholar]
- Chong P. L., Tang D., Sugar I. P. Exploration of physical principles underlying lipid regular distribution: effects of pressure, temperature, and radius of curvature on E/M dips in pyrene-labeled PC/DMPC binary mixtures. Biophys J. 1994 Jun;66(6):2029–2038. doi: 10.1016/S0006-3495(94)80996-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyslop P. A., Morel B., Sauerheber R. D. Organization and interaction of cholesterol and phosphatidylcholine in model bilayer membranes. Biochemistry. 1990 Jan 30;29(4):1025–1038. doi: 10.1021/bi00456a027. [DOI] [PubMed] [Google Scholar]
- Lentz B. R., Barrow D. A., Hoechli M. Cholesterol-phosphatidylcholine interactions in multilamellar vesicles. Biochemistry. 1980 Apr 29;19(9):1943–1954. doi: 10.1021/bi00550a034. [DOI] [PubMed] [Google Scholar]
- Mabrey S., Mateo P. L., Sturtevant J. M. High-sensitivity scanning calorimetric study of mixtures of cholesterol with dimyristoyl- and dipalmitoylphosphatidylcholines. Biochemistry. 1978 Jun 13;17(12):2464–2468. doi: 10.1021/bi00605a034. [DOI] [PubMed] [Google Scholar]
- Melchior D. L., Scavitto F. J., Steim J. M. Dilatometry of dipalmitoyllecithin-cholesterol bilayers. Biochemistry. 1980 Oct 14;19(21):4828–4834. doi: 10.1021/bi00562a018. [DOI] [PubMed] [Google Scholar]
- Muczynski K. A., Stahl W. L. Incorporation of danyslated phospholipids and dehydroergosterol into membranes using a phospholipid exchange protein. Biochemistry. 1983 Dec 6;22(25):6037–6048. doi: 10.1021/bi00294a052. [DOI] [PubMed] [Google Scholar]
- Needham D., Nunn R. S. Elastic deformation and failure of lipid bilayer membranes containing cholesterol. Biophys J. 1990 Oct;58(4):997–1009. doi: 10.1016/S0006-3495(90)82444-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers W., Glaser M. Characterization of lipid domains in erythrocyte membranes. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1364–1368. doi: 10.1073/pnas.88.4.1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers J., Lee A. G., Wilton D. C. The organisation of cholesterol and ergosterol in lipid bilayers based on studies using non-perturbing fluorescent sterol probes. Biochim Biophys Acta. 1979 Mar 23;552(1):23–37. doi: 10.1016/0005-2736(79)90243-8. [DOI] [PubMed] [Google Scholar]
- Schroeder F., Barenholz Y., Gratton E., Thompson T. E. A fluorescence study of dehydroergosterol in phosphatidylcholine bilayer vesicles. Biochemistry. 1987 May 5;26(9):2441–2448. doi: 10.1021/bi00383a007. [DOI] [PubMed] [Google Scholar]
- Schroeder F., Jefferson J. R., Kier A. B., Knittel J., Scallen T. J., Wood W. G., Hapala I. Membrane cholesterol dynamics: cholesterol domains and kinetic pools. Proc Soc Exp Biol Med. 1991 Mar;196(3):235–252. doi: 10.3181/00379727-196-43185. [DOI] [PubMed] [Google Scholar]
- Snyder B., Freire E. Compositional domain structure in phosphatidylcholine--cholesterol and sphingomyelin--cholesterol bilayers. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4055–4059. doi: 10.1073/pnas.77.7.4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerharju P. J., Virtanen J. A., Eklund K. K., Vainio P., Kinnunen P. K. 1-Palmitoyl-2-pyrenedecanoyl glycerophospholipids as membrane probes: evidence for regular distribution in liquid-crystalline phosphatidylcholine bilayers. Biochemistry. 1985 May 21;24(11):2773–2781. doi: 10.1021/bi00332a027. [DOI] [PubMed] [Google Scholar]
- Tang D., Chong P. L. E/M dips. Evidence for lipids regularly distributed into hexagonal super-lattices in pyrene-PC/DMPC binary mixtures at specific concentrations. Biophys J. 1992 Oct;63(4):903–910. doi: 10.1016/S0006-3495(92)81672-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Dreele P. H. Estimation of lateral species separation from phase transitions in nonideal two-dimensional lipid mixtures. Biochemistry. 1978 Sep 19;17(19):3939–3943. doi: 10.1021/bi00612a009. [DOI] [PubMed] [Google Scholar]


