Fig 13. EBNA3A and EBNA3C together modulate the activity of multiple cell cycle inhibitors by more than one mechanism.
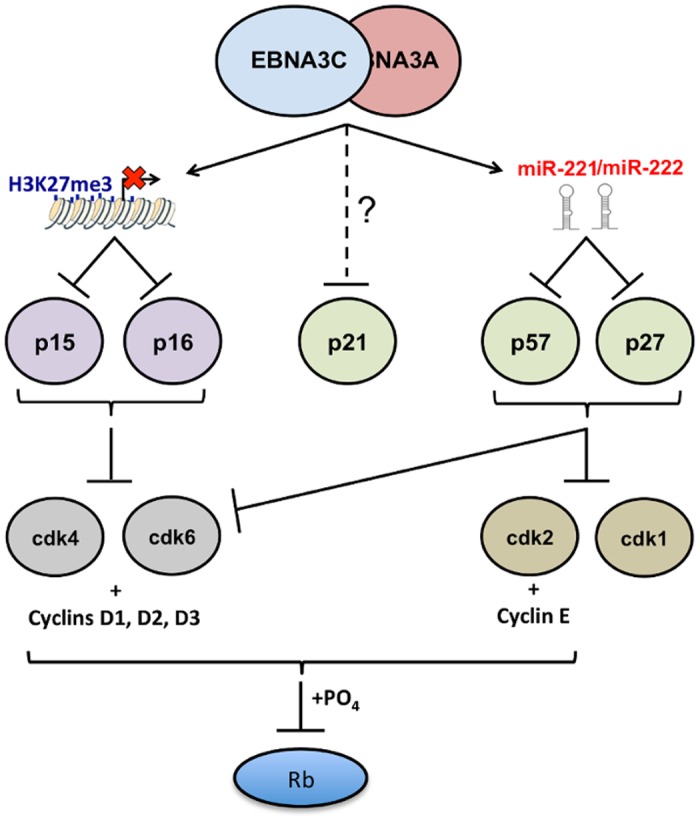
Schematic representation of how, by acting together, EBNA3A and EBNA3C regulate members of two families of cyclin-dependent kinase inhibitors (CDKIs). EBNA3A and EBNA3C jointly repress expression of the two closely related INK4 CDKIs p16INK4a (encoded by CDKN2A) and p15INK4b (encoded by CDKN2B). In both cases repression of transcription is associated with the tethering of EBNA3A and EBNA3C to chromatin in the region of the CDKN2A/CDKN2B locus [19,30,32]. Inhibition of transcription involves the polycomb-mediated epigenetic histone modification H3K27me3. In this report the same combination of EBNA3 proteins has been shown to repress two members of the second (CIP/KIP) family of CDKIs. Here repression of protein expression is not directed at the transcription of the genes for p57KIP2 (CDKN1C) and p27KIP1 (CDKN1B), but is post-transcriptional and mediated by the miR-221/miR-222 cluster that is directly transactivated by EBNA3A and EBNA3C. The biochemically related proteins p16INK4a and p15INK4b both have the capacity to inhibit CDK4 and CDK6 activity. The CIP/KIP kinases are more promiscuous and target, in addition, other CDKs including CDK2. Recently there have been reports that EBNA3A and EBNA3C can each also regulate the third member of the CIP/KIP family, namely p21CIP1 [85,86] but the mechanisms in LCLs have not been clearly defined.
