Fig 3.
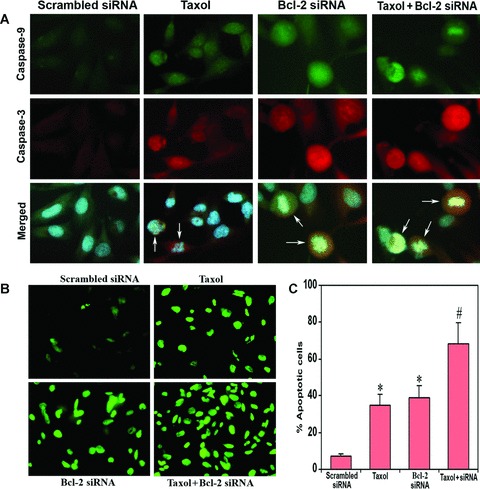
In situ stainings for biochemical markers of apoptosis in U251MG cells. Treatment (72 hrs) of cells: transfection with a plasmid vector expressing scrambled siRNA (treated control), 100 nM taxol, transfection with a plasmid vector expressing Bcl-2 siRNA and taxol + Bcl-2 siRNA. (A) Double immunofluorescent stainings to examine active fragments of caspase-9 and caspase-3. The cells were incubated overnight at 4°C with specific antibodies for active fragments of caspase-9 and caspase-3, washed and then treated with FITC conjugated and Texas red conjugated secondary antibodies at room temperature for 1 hr. Hoechst 33342 was used to counterstain the nucleus. Merged microphotographs demonstrated simultaneous expression of active fragments of caspase-9 and caspase-3 as well as disintegration of nucleus in the apoptotic cells (shown with arrows). (B) Fluorescent TUNEL assay for detection of apoptotic cells. Treatment with combination of taxol and Bcl-2 siRNA resulted in more apoptotic cell death than either treatment alone. (C) Quantitation of TUNEL-positive cells. Data are representative of four independent experiments (*P < 0.001 when compared with the scrambled siRNA treatment mean values and #P < 0.001 when compared with taxol or Bcl-2 siRNA treatment mean values).
