Abstract
Dopaminergic signaling pathways are conserved between mammals and Drosophila, but the factors important for maintaining the functional pool of synaptic dopamine are not fully understood in Drosophila. In this study, we characterized the releasable and reserve dopamine pools in Drosophila larvae using ATP/ P2X2-mediated stimulation. Dopamine release was stable with stimulations performed at least every 5 min but decayed with stimulations performed 2 min apart or less, indicating the replenishment of the releasable pool occurred on a time scale between 2 and 5 min. Dopamine synthesis or uptake were pharmacologically inhibited with 3-iodotyrosine and cocaine, respectively, to evaluate their contributions to maintaining the releasable dopamine pool. We found that both synthesis and uptake were needed to maintain the releasable dopamine pool, with synthesis playing a major part in long-term replenishment and uptake being more important for short-term replenishment. These effects of synthesis and uptake on different time scales in Drosophila are analogous to mammals. However, unlike in mammals, cocaine did not activate a reserve pool of dopamine in Drosophila when using P2X2 stimulations. Our study shows that both synthesis and uptake replenish the releasable pool, providing a better understanding of dopamine regulation in Drosophila.
Introduction
Dopamine signaling plays a major role in a variety of brain functions, including emotion, reward, cognition, memory, learning, locomotion and motor control (Schultz 2007, Grace et al. 2007). In the central nervous system, dopamine is released by exocytosis after an action potential and acts in the extracellular space as a neurotransmitter. The amount of dopamine available for exocytosis determines the functional pool. Two main sources contributing to the releasable pool are newly synthesized dopamine and dopamine that is recycled from the extracellular space through uptake by the dopamine transporter (Venton and Wightman 2003). Understanding dopamine regulation is essential for the treatment of many neurological and psychiatric diseases such as Parkinson disease, Huntington disease, schizophrenia, and drug addiction.
Dopamine pools have been studied extensively in mammalian models; however, Drosophila melanogaster, the fruit fly, is an attractive model organism because of its simple nerve system, relatively short life cycle, and ease of molecular and genetic manipulation (Nichols 2006). While genetically altered mice can take years to make, Drosophila genetic models can be produced in a few months. A variety of sophisticated genetic manipulations have been developed for Drosophila, allowing large-scale screening of mutants to model some aspects of human diseases (Nichols 2006, Bier 2005). Our lab has developed methods for directly measuring dopamine in Drosophila and has verified that dopamine regulatory functions such as synthesis, uptake, and vesicular release are conserved between Drosophila and mammals (Vickrey et al. 2009, Vickrey et al. 2013). However, the factors important for maintaining the releasable dopamine pool in Drosophila are not fully understood.
Taking advantage of fly genetics, several neural excitation methods with genetically encoded triggers have been successfully used in Drosophila (Venken et al. 2011). Among these, ATP/P2X2-mediated stimulation has become an elegant method for targeted control of neuronal activity. P2X2 is a member of the ligand-gated cation channel P2X family which is activated by extracellular ATP. P2X2 undergoes three sequential ATP binding steps in a cooperative manner (Ding and Sachs 1999). Once fully bound, the channel opens rapidly and an inward flow of cations leads to neuronal excitation (North 2002). A distinguishing characteristic of this channel is its slow desensitization, as currents at P2X2 receptors decline little during sustained ATP application of a few seconds (Brake et al. 1994, Collo et al. 1996). This feature makes it more suitable for inducing large amounts of neurotransmitter release compared to other cation channels. The Drosophila genome does not encode a P2X2 homolog (Littleton and Ganetzky 2000) and previous studies suggest that there are no acute behavioral or physiological effects of ATP in the absence of transgenic P2X2 in Drosophila (Lima and Miesenbock 2005, Yao et al. 2012). Thus, through genetic modification, P2X2 can be inserted into specific neurons and with exogenously applied ATP, those P2X2-expressing neurons can be excited. ATP/P2X2-mediated stimulation for target neural excitation has been established in both larval and adult fly nervous systems during behavioral and electrophysiology experiments (Huang et al. 2010, Hu et al. 2010, Yao et al. 2012), but no ATP/P2X2-mediated neurotransmitter release has been directly detected in Drosophila.
In this study, we used ATP/P2X2-mediated dopamine release to evaluate the roles of synthesis and uptake in maintaining the releasable dopamine pool in Drosophila. Dopamine release was measured with fast-scan cyclic voltammetry (FSCV) at a carbon-fiber microelectrode implanted in the neruopil of an isolated larval fly ventral nerve cord (VNC), which was genetically modified to express P2X2 in dopaminergic cells. ATP was applied at various intervals with synthesis or uptake pharmacologically inhibited to evaluate the recovery of the releasable pool. We found that both synthesis and uptake are needed to maintain the releasable dopamine pool, with synthesis playing a major part in long-term replenishment and uptake being more important for short-term replenishment. In mice, cocaine can increase dopamine release by activating a synapsin-dependent reserve pool of dopamine (Venton et al. 2006, Kile et al. 2010) However, no similar activation of a reserve pool was observed after cocaine in Drosophila with P2X2 stimulation. Our study facilitates a better understanding of dopamine regulation in Drosophila, strengthening this model organism for the study of dopaminergic diseases.
Experimental procedures
Chemicals
All chemicals were purchased from Sigma-Aldrich (St. Louis, MO). Solutions were made with Milli Q water (Millipore, Billerica, MA). Electrode calibrations and Drosophila dissections and experiments were conducted in phosphate buffer (131.25 mM NaCl, 3.0 mM KCl, 10.0 mM NaH2PO4, 1.2 mM MgCl2, 2.0 mM Na2SO4, and 1.2 mM CaCl2) with 11.1 mM glucose, 5.3 mM trehalose and pH adjusted to 7.4. ATP solutions to stimulate release, ranging from 0.2 to 1 mM, were made with phosphate buffer. Stock solutions (1 mM) of cocaine and 3-iodotyrosine were made in water and the final concentration in the bath around the Drosophila VNC was 60 μM cocaine and 100 μM 3-iodotyrosine.
Preparation of Ventral Nerve Cords
Flies containing UAS-P2X2 on the third chromosome (a gift from Jayaraman Lab, Janelia Farm Research Campus) were crossed with flies containing th-GAL4 on the second chromosome (a gift from J. Hirsh, University of Virginia) to generate a heterozygous line. The fly dissection and all measurements were performed at room temperature. The central nervous system of a 5-day-old wandering third instar larva (L3W) was dissected out in phosphate buffer. The optic lobes were removed by a horizontal cut across the anterior thorax region to yield an isolated ventral nerve cord (VNC) and then an additional horizontal cut was made at the posterior-most portion of the ventral nerve cord to facilitate micropipette insertion. The isolated VNC was adhered neuropil side down onto the bottom of a Petri dish with 3 mL of buffer. The VNC was visualized under a 40× water immersion objective of a microscope (Carl Zeiss Microscopy, LLC). Using a micromanipulator, an electrode was implanted into the VNC four to six segments away from the cut edge and a picospritzing micropipette was inserted 15-20 μm away from the electrode. The electrode and micropipette were allowed to equilibrate after implantation for 10 min prior to data collection. Ten seconds of baseline data were collected before each stimulation.
Electrochemical Measurements
Cylindrical carbon-fiber microelectrodes were fabricated in house from T-650 carbon fibers (a gift of Cytec Engineering Materials, West Patterson, NJ) as previously described (Swamy and Venton 2007). Fast-scan cyclic voltammetry were performed using a ChemClamp potentiostat (Dagan, Minneapolis, MN, n = 0.01 headstage), PCI 6711 and 6052 computer interface cards (National Instruments, Austin, TX) and a home-built breakout box. Data collection was computer controlled by the TarHeel CV software program (gift of Mark Wightman, University of North Carolina). The electrode was scanned from −0.4 to 1.3 V and back at a scan rate of 400 V/s every 100 ms vs a Ag/AgCl reference electrode. Electrodes were calibrated with 1 μM dopamine before and after use in situ. For drug experiments, a second calibration was conducted in the presence of drug to account for possible drug effects on the electrode sensitivity.
Picospritzing micropipettes were made by pulling a 1.2 mm × 0.68 mm glass capillary (A-M Systems, Carlsburg, WA) using a vertical pipette puller (Narishige, Japan). The tip of the micropipette was trimmed to make an opening. Micropipettes were filled with ATP solutions ranging from 0.2 to 1 mM, and ATP was pressure ejected with a Picospritzer III instrument (Parker Hannifin, Fairfield, NJ). The pipette was calibrated by ejecting ATP solution into oil and measuring the diameter of the ejected droplet to calculate the volume (volume = 1/6πd3).
Statistics and Data Analysis
All values are presented as mean ± standard error of the mean (SEM) for n number of fly samples and all error bars are given as SEM. All statistics were performed in GraphPad Prism (GraphPad Software,Inc., La Jolla, CA) and significance was considered at the 95% confidence level (p < 0.05). Paired t-tests were performed to compare data before and after drugs in the same sample and unpaired t-tests were used to compare data in two different groups. A one-way ANOVA with Bonferroni post-tests was performed to evaluate the effect of the amount of ATP on stimulated dopamine release. A two-way ANOVA with Bonferroni post-test was used to evaluate stimulation interval and drug effects during repeated stimulations.
Results
Characterizing ATP/P2X2 mediated dopamine release in Drosophila
To provide neuron-specific stimulation, we expressed P2X2 in dopaminergic neurons containing tyrosine hydroxylase using the yeast-based GAL4/UAS system. A microelectrode was implanted into the neuropil of a larval VNC and a capillary micropipette filled with ATP was inserted from the other side approximately 15–20 μm away from the electrode. Picoliter volumes of ATP were pressure-ejected into the neuropil, and the dopamine response was monitored with FSCV. Fig. 1A shows that injection of 2 pmol ATP into a th-GAL4; UAS-P2X2 larval VNC elicits dopamine release. The large green and blue areas on the color plot correspond to the oxidation and reduction of dopamine and the characteristic oxidation and reduction peaks for dopamine are also observed in the background-subtracted cyclic voltammogram. The dopamine response is slightly delayed after ATP injection, primarily because ATP has to diffuse from the micropipette to the area of the microelectrode where stimulated dopamine can be detected. Upon ATP injection, there are small current fluctuations close to switching potential (1.3 V) and at the beginning of the CV (around -0.4 to 0 V), which are also seen in a larval VNC without P2X2 (Fig. 1B). These small changes are likely due to changes in the background current caused by pressure changes and these changes can cause a small dip in the concentration over time traces for dopamine at the time of injection. In control larval VNCs that do not express P2X2, no dopamine response is observed when ATP is injected (Fig. 1B). Although ATP is also an electroactive molecule, no characteristic peaks for ATP are detected in tissue, because the oxidation potential for ATP is around 1.4 V (Ross and Venton 2012), and the waveform does not scan high enough to detect ATP. In the future, we could modify the waveform to scan to 1.45 V if we wanted to quantify the amount of ATP that reaches the electrode.
Figure 1.
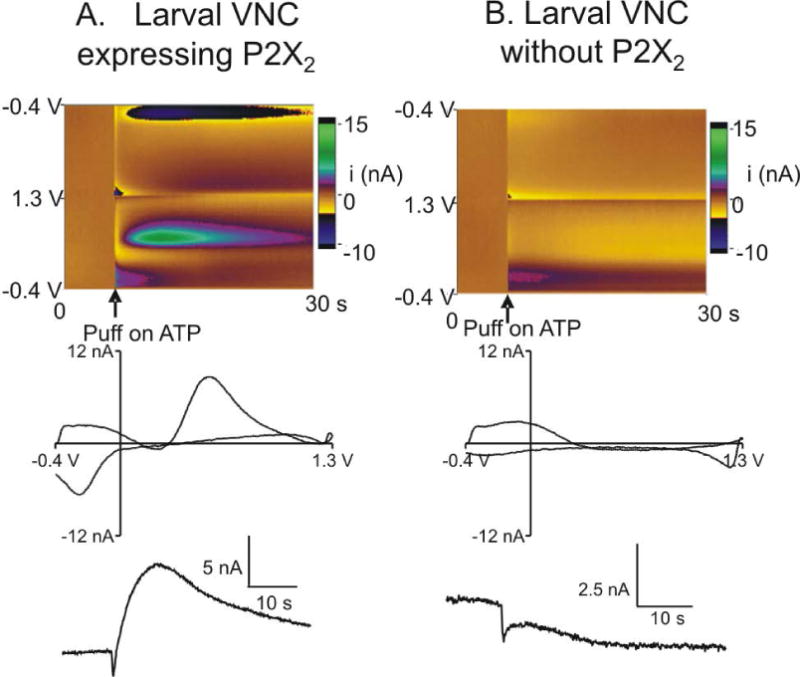
Characterization of ATP evoked dopamine signal in P2X2 flies. Top row: false color plots with time on the x-axis, applied voltage on the y-axis and Faradaic current in pseudo-color. Injection of ATP is denoted by the arrow under the figure. Middle row: background-subtracted cyclic voltammograms. Bottom row: signal traces show dopamine current changes over time. A.) 2 pmol ATP was injected into a larval VNC expressing P2X2 and the color plot and CV show that dopamine is released upon ATP stimulation. B.) 2 pmol ATP was injected into a control larval VNC without P2X2 expression and the color plot shows minor fluctuations upon ATP injection corresponding to pressure changes, but the CV does not show any characteristic dopamine peaks.
Next, we tested the dependence of peak dopamine concentration on the amount of ATP injected (Fig. 2). The data show a non-significant trend that the dopamine release increases as the amount of ATP increases until saturation is reached around 2 pmol ATP (One way ANOVA, F[6,42] = 1.542, p=0.189). At that amount of ATP, approximately 0.4 μM of dopamine is released. Thus, 2 pmol of ATP was applied for the rest of the experiments.
Figure 2.
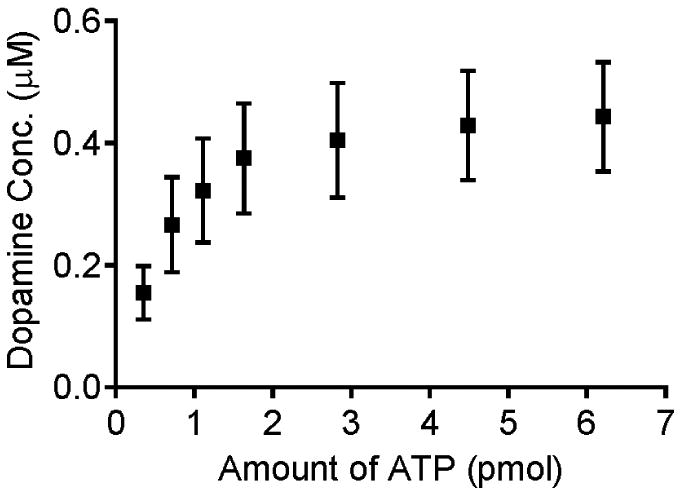
Effect of the amount of pressure-injected ATP on evoked dopamine release. Data are mean ± SEM with n=7 samples. The pipette was filled with 200 μM ATP and placed 15-20 μm away from the electrode. Different volumes were pressure ejected to control the amount. The data show a non-significant trend that the dopamine concentration increases with the amount of ATP applied until a plateau is reached around 2 pmol applied (one-way ANOVA, F[6,42] = 1.542, p=0.189).
Effect of stimulation intervals on dopamine release
The stability of ATP/P2X2 induced dopamine release during multiple stimulations was tested (Fig. 3) by repeating stimulations at 0.5, 1, 2, 5 or 10 min intervals. For each animal, data were normalized to the peak concentration of the first stimulation to control for sample variability. Two-way ANOVA analysis shows a significant interaction of stimulation interval and stimulation number (F[28,220] = 5.291, p<0.0001) and significant main effects of stimulation interval (F[4,220] = 236.7, p<0.0001) and stimulation number (F[7,220] = 14.98, p<0.0001). Dopamine release decayed when stimulations were performed at 0.5, 1 or 2 min intervals but is stable at 5 or 10 min intervals, suggesting the replenishment of the releasable dopamine pool occurs between 2 and 5 min. For stimulations intervals of less than 2 min, dopamine release decreased sharply after the initial stimulation, and then decayed to a stable low level. On the second stimulation, the signal is 26%, 57% and 68% of the initial value for 0.5, 1 and 2 min intervals, respectively. The dopamine concentration for this second stimulation for the 0.5 min interval is significantly lower than that of the 1 and 2 min interval, while the difference between the 1 and 2 min interval is not significant (Bonferroni post-test, see Table S1 for all statistics). We focused our analysis on the first five stimulations for pharmacological experiments.
Figure 3.
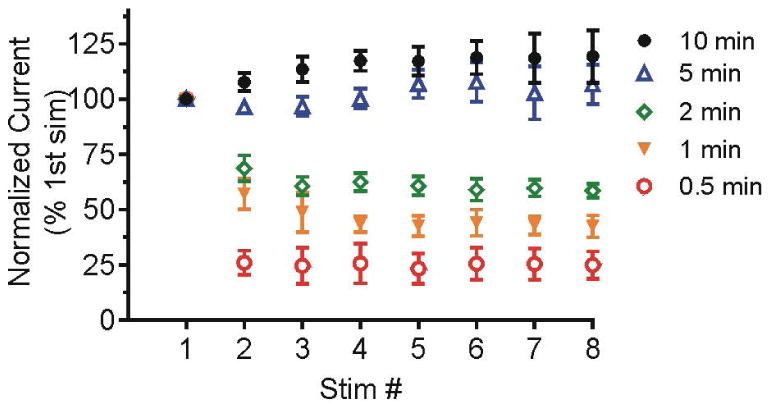
Dopamine release during stimulations repeated at 0.5, 1, 2, 5 or 10 min intervals. 2 pmol ATP was pressure injected and the pipette tip was 15-20 μm away from the electrode. Data are normalized to the peak concentration of the initial stimulation for each animal and are presented as mean ± SEM (n= 6-7). Dopamine release decays when stimulations are performed at 0.5, 1 and 2 min interval but is stable at 5 or 10 min interval.
Effect of synthesis inhibition and uptake inhibition on stimulated release
To evaluate the role and time course of synthesis and uptake in replenishing the releasable dopamine pool, dopamine synthesis or uptake was pharmacologically inhibited during repeated stimulations at 1 or 5 min intervals. Dopamine uptake was inhibited with 60 μM cocaine, a known dopamine transporter inhibitor (Greco and Garris 2003). Cocaine significantly prolongs evoked dopamine signaling in Drosophila larvae (Vickrey et al. 2009, Vickrey et al. 2013) and slows clearance of exogenous dopamine in Drosophila adults (Makos et al. 2009). Dopamine synthesis was inhibited by 100 μM 3-iodotyrosine. 3-iodotyrosine inhibits the activity of tyrosine hydroxylase, the rate-limiting enzyme in dopamine synthesis and significantly decreased steady-state amounts of dopamine after 2 days of feeding to Drosophila larvae (Neckameyer 1996).
To test the effects of drug without repeated stimulations, we stimulated dopamine release in buffer first and then allowed 5 min for the dopamine pool to fully recover. After that we applied drug and waited 15 min before stimulating again. The dopamine signaling was compared before and after drug application. Figure 4 shows an example trace of a stimulation before and after cocaine or 3-iodotyrosine. After cocaine application, there was a significant increase in evoked dopamine concentration (paired t test, n=16, p<0.0001) and the time to half decay (t50) (paired t test, n=16, p<0.0001), due to inhibition of dopamine uptake. The first stimulation after 3-iodotyrosine has no significant change in dopamine concentration or t50 value (paired t-test, n=14, p= 0.2484 for concentration and p= 0.2508 for t50). Thus, the releasable pool is not depleted after 3-iodotyrosine on the first stimulation.
Figure 4.
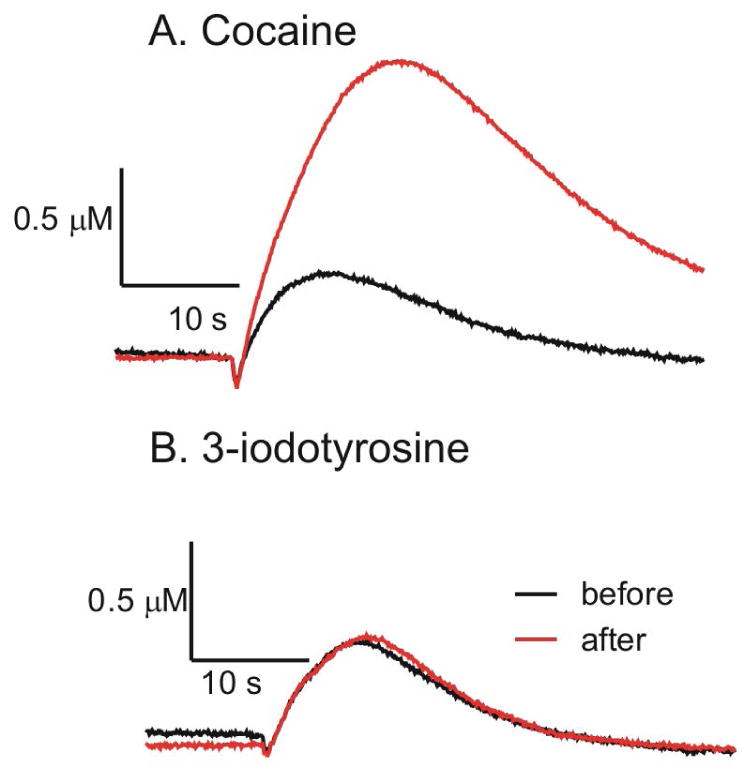
Concentration vs time traces for dopamine after ATP (2 pmol)/P2X2 stimulation before and the first stimulation after drugs. A.) Before and 15 min. after incubation in 60 μM cocaine. B.) Before and after 15 min incubation in 100 μM 3-iodotyrosine.
Figure 5 shows example traces of the effects of cocaine and 3-iodotyrosine incubation on dopamine release during repeated stimulations performed at 1 and 5 min intervals. The first and fifth stimulations are compared. Stimulations performed in buffer are stable with a 5 min stimulation interval but decrease with a 1 min interval (Fig. 5A,D). When uptake is blocked by cocaine, repeated stimulations at 1 min intervals deplete the dopamine release by the 5th stimulation (Fig. 5B). However, due to continued synthesis of new dopamine, cocaine blockade of the dopamine transporter only partially depletes the releasable dopamine pool when stimulations are performed at 5 minute intervals (Fig. 5E). The depletion effect of 3-iodotyrosine is about the same for 1 min and 5 min stimulation intervals (Fig. 5C, F).
Figure 5.
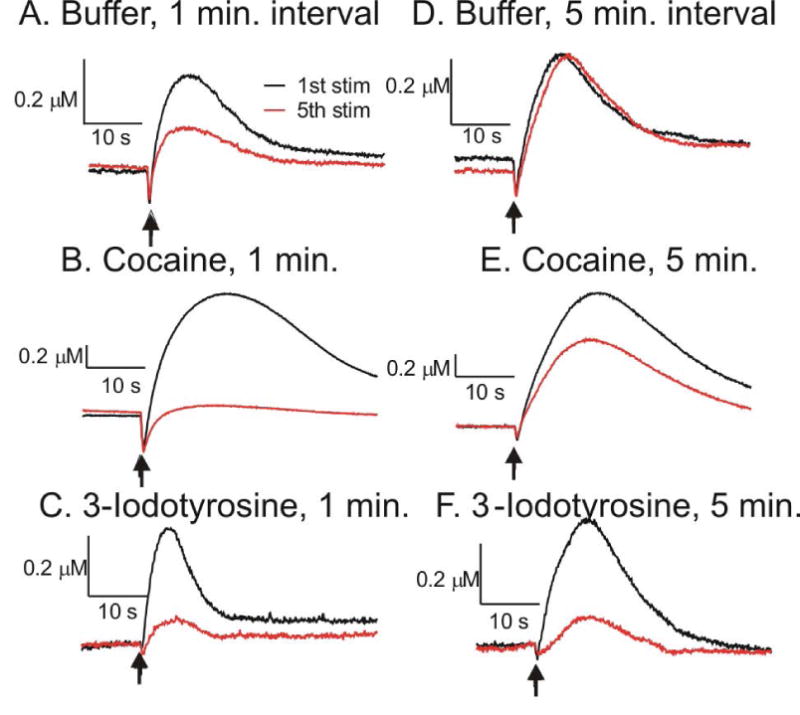
Effect of buffer, cocaine and 3-iodotyrosine on repeated stimulations. The representative concentrations vs time profiles are shown for the first stimulation (black) and the fifth stimulation (red). A.) Incubation in buffer, 1 min stimulation interval. B.) Incubation in 60 μM cocaine, 1 min stimulation interval. C.) Incubation in 100 μM 3-iodotyrosine, 1 min stimulation interval. D.) Incubation in buffer, 5 min stimulation interval. E.) Incubation in 60 μM cocaine, 5 min stimulation interval. F.) Incubation in 100 μM 3-iodotyrosine, 5 min stimulation interval. The dopamine concentration is larger and the time response is slower in cocaine at the 1st stimulation, while there is no difference in 3-iodotyrosine. Note the concentration scale for cocaine is different from the buffer and 3-iodotyrosine groups.
Fig. 6 shows the averaged effects of cocaine and 3-iodotyrosine on dopamine release, with each animal normalized to its first stimulation. For the 1 min interval stimulation, two-way ANOVA shows a significant interaction of stimulation number and drug (F[14,168] = 3.47, p<0.0001) and significant main effect of stimulation number (F[7,168] = 86.58, p<0.0001) and drug (F[2,168] = 67.61, p<0.0001). The dopamine concentration decays significantly more in the cocaine group than in buffer (Bonferroni post-test, see Table S2 for all statistics). At the second stimulation, the dopamine release decreased to 15 ± 3% of the initial value in cocaine compared to 57 ± 7% in buffer. With 1 min intervals, there is no significant difference in the decay of dopamine concentration in 3-iodotyrosine or in buffer. For the 5 min interval stimulation, there is also a significant interaction of stimulation number and drug (F[14,121] = 11.77, p<0.0001) and significant main effect of stimulation number (F[7,121] = 24.26, p<0.0001) and drug (F[2,121] = 405.17, p<0.0001) (Fig. 6B). While the release in buffer is stable, there is a significant decay with repeated stimulations in both the cocaine and 3-iodotyrosine, with the 3-iodotyrosine group decaying significantly more (Bonferroni post-test, see Table S2 for all statistics). For the second stimulation, dopamine release in 3-iodotyrosine is 49 ± 5 % of the initial value compared to 68 ± 3% in cocaine.
Figure 6.
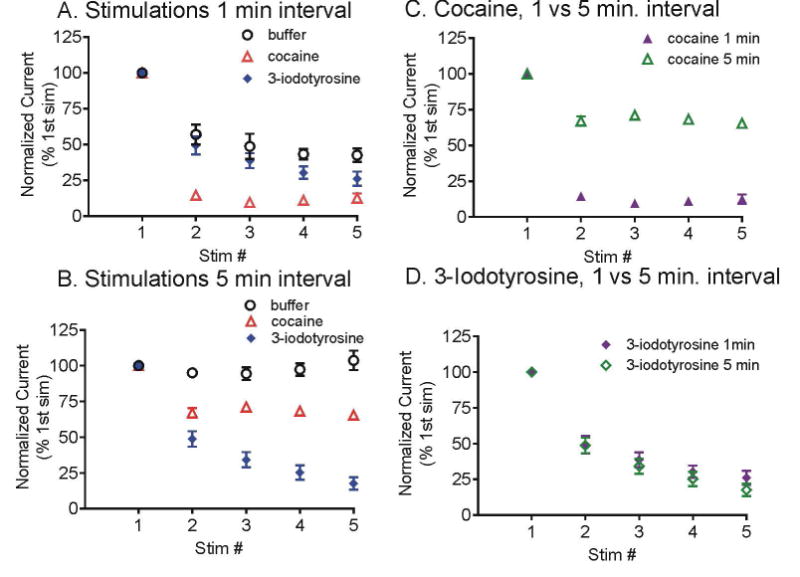
Effect of cocaine and 3-iodotyrosine on repeated stimulations. Stimulations were performed every A.) 1 min or B.) 5 min. Dopamine reuptake was inhibited by 60 μM cocaine (red triangles) or synthesis was inhibited by 100 μM 3-iodotyrosine (blue diamonds). Control samples in buffer are black circles. 2 pmol ATP was pressure injected and the pipette tip was 15-20 μm away from the electrode. Data are normalized to the peak concentration of the initial stimulation for each animal and are presented as mean ± SEM, n= 5-7. C.) Data for cocaine at 1 min interval (purple closed triangles) or 5 min intervals (green open triangles) are shown on one graph. Dopamine depletion is greater for the 1 min intervals. D.) Data for 3-iodotyrosine at 1 min interval (purple closed diamonds) or 5 min intervals (green open diamonds) are shown on one graph. The amount of dopamine depletion is about the same.
Fig. 6 C and D show the data for the 1 and 5 min stimulation intervals on the same graph for cocaine and 3-iodotyrosine. For stimulations performed at 1 or 5 min interval, the decay is very similar with 3-iodotyrosine, while with cocaine, the decay at 1 min interval is significantly more than at 5 min interval (two way ANOVA with Bonferroni post-test, see Table S3). Therefore, cocaine and 3-iodotyrosine have different effects on dopamine release based on the interval during repeated stimulations.
Investigating potential cocaine-activated reserve dopamine pool in Drosophila
In mammals, cocaine increases the extracellular dopamine level by inhibiting uptake but also by increasing dopamine release (Stamford et al. 1989, Lee et al. 2001). Cocaine can augment dopamine release after depletion of the readily releasable pool by activating release of a synapsin-mediated vesicle reserve pool (Venton et al. 2006, Kile et al. 2010). Here, we investigated if a cocaine-sensitive reserve pool existed in Drosophila. Dopamine synthesis was inhibited with 100 μM 3-iodotyrosine and the releasable pool was depleted with consecutive stimulations. Fig. 7A shows that after 8 simulations at 1 min intervals, the dopamine concentration decayed from 0.37 ± 0.07 μM to 0.05 ± 0.01 μM. Then the sample was incubated with 60 μM cocaine for 15 min and another four stimulations were performed. The dopamine signal increased to 0.19 ± 0.02 μM after cocaine application, and then progressively decayed to 0.04 ± 0.01 μM on the last stimulation. The concentration vs time traces are wider and t50 increases after cocaine, indicating that cocaine did block uptake. A separate control group was conducted with the same stimulation parameters, except that the sample was incubated in buffer for 15 min after the depletion of releasable pool (Fig. 7B). A similar trend was observed in the control group. The dopamine concentration decreased from 0.63 ± 0.09 μM to 0.05 ± 0.02 μM after 8 stimulations, then increased to 0.20 ± 0.01 μM after 15 min incubation in buffer, and progressively decayed to 0.03 ± 0.01 μM on the last stimulation. Thus, there is no significant difference in dopamine release between the cocaine or buffer group after the 15 min incubation (unpaired t test, p= 0.8306).
Figure 7.
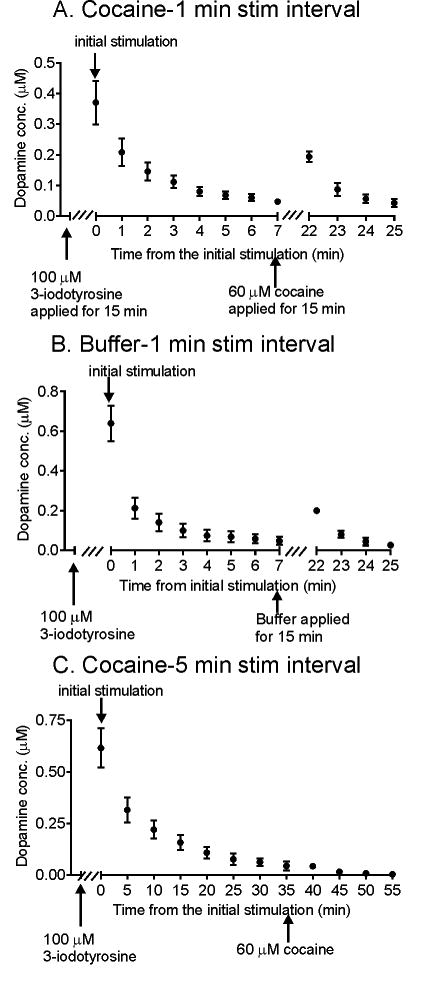
Effect of cocaine on dopamine release after depletion of the releasable pool. A.) Eight consecutive stimulations were performed at 1 min intervals after 100 μM 3-iodotyrosine was applied for 15 min. Then, 60 μM cocaine was applied for 15 min, and another four stimulations were performed at 1 min interval (n=8). B.) Eight consecutive stimulations were performed at 1 min intervals after 100 μM 3-iodotyrosine was applied for 15 min. Then buffer was applied instead of cocaine for 15 min and 4 stimulations performed (n=5). The trends are the same for cocaine and buffer. C.) Eight consecutive stimulations were performed at 5 min intervals after 100 μM 3-iodotyrosine was applied for 15 min. Then, 60 μM cocaine was applied and 4 more stimulations applied every 5 min. (n=6). Data are mean ± SEM.
It is possible that this dose of 3-iodotyrosine did not fully block synthesis and thus some of the releasable pool was replenished during the 15 min incubation time when no stimulations were performed. Furthermore, cocaine may take a few minutes to have an effect and uptake may replenish the pool during the initial part of the 15 min incubation time. To address this increase in release after the 15 min incubation without stimulation in cocaine, we also performed the same experiment with 5 min stimulation intervals (Fig. 7C). Stimulations were performed every 5 min after application of 3-iodotyrosine and cocaine, and there is no increase in release after cocaine. Ten min after cocaine, there is no detectable dopamine signal that can be measured, as dopamine is completely depleted. Thus, our results indicate that cocaine does not significantly increase dopamine release with ATP/P2X2 stimulations after depletion of the readily releasable pool.
Discussion
In this paper, we evaluated the importance of synthesis and reuptake for maintaining the releasable dopamine pool in Drosophila using ATP/P2X2-mediated release. The releasable dopamine pool was maintained by both synthesis and uptake, with synthesis more important for long-term replenishment and uptake more important for short-term replenishment. While cocaine is reported to elevate dopamine release by mobilizing a synapsin-dependent reserve pool in rodents, there was no cocaine-activated reserve pool of dopamine in Drosophila using ATP/P2X2-mediated release. Therefore, Drosophila releasable dopamine pools are regulated by synthesis and uptake, similar to mammals, but they do not have a cocaine-activated storage pool like mammals.
P2X2-mediated release of dopamine
While several groups have used electrophysiology studies to confirm the ability of ATP/P2X2 to stimulate targeted neurons (Huang et al. 2010, Hu et al. 2010), this is the first time that ATP/P2X2 stimulated neurotransmitter release has been directly monitored in Drosophila. The amount of dopamine released plateaued at 0.4 μM with around 2 pmol ATP, indicating that P2X2 channels have been saturated or the releasable pool of dopamine was depleted. The concentration of ATP we used in the pipette (200 uM) was much lower than many previous studies where a typical 1 mM ATP or higher was used (Huang et al. 2010, Hu et al. 2010, Yao et al. 2012). Thus, a modest concentration of ATP is enough to cause maximal dopamine release.
Previously, optically-stimulated dopamine release has been measured in flies which are genetically modified to express Channelrhodopsin-2 (ChR2), a blue-light activated cation channel in dopaminergic neurons (Vickrey et al. 2009, Xiao et al. 2014). Seven-second long stimulations resulted in 0.81 ± 0.06 μM of dopamine in those flies (Vickrey et al. 2009), which is higher than that evoked by ATP/P2X2 in this study. However, the duration of the ATP/P2X2 stimulations is longer. For repeated stimulations, the lower amounts of dopamine measured might mean that pools are not depleted as much as with stronger stimulations. Future studies could compare the effects of optogenetic and ATP/P2X2 stimulations on repeated stimulations. We used heterozygote flies with only one copy of the P2X2 gene and thus the expression density of the P2X2 channel may be lower compared to the ChR2 flies, which are homozygous and have two copies of ChR2. Also, the ion flux may be different for the P2X2 and ChR2 channels. Future studies with flies homozygous in P2X2 may have higher dopamine release, but the ability to stimulate release with only one copy of P2X2 makes it useful for genetic studies in combination with other genetic mutants. Furthermore, the ATP/P2X2-mediated release is suitable for measurements in brain regions, such as deeper neuropil in the adult fly, where light penetration through the tissue is difficult.
The releasable dopamine pool is maintained by both synthesis and uptake
Dopamine release was stable when stimulations were performed at least every 5 min, while the signal decayed with stimulations performed 2 min apart, indicating the replenishment of the releasable dopamine pool occurred on a time scale between 2 and 5 min. Two main sources for maintaining the releasable pool are newly synthesized dopamine and dopamine that is recycled by the dopamine transporter. To investigate the contribution of synthesis and uptake to the recovery of the releasable dopamine pool, pharmacological experiments were performed. During closely repeated stimulations (1 min apart), dopamine release decreased faster when uptake was blocked, while the decay with synthesis inhibition was similar to that in buffer. For the first two stimulations performed 1 min apart, dopamine release decreased by 85 ± 3 % in cocaine compared to by 43 ± 7 % in buffer (Fig. 6A). Therefore, on a short time scale, uptake is responsible for maintaining about 40% of the releasable pool and synthesis does not have a measurable effect. With 5 min interval stimulations, dopamine release was stable in buffer, while the signal decreased by 51 ± 5% in 3-iodotyrosine and 32 ± 3% in cocaine at the second stimulation (Fig. 6B). Thus, on the longer time scale, newly synthesized dopamine makes up about 50% and recycled dopamine about 30% of the releasable pool. Because these numbers do not add up to 100 %, there must be additional sources of dopamine, including dopamine that is already synthesized and waiting to be packaged into vesicles or dopamine in vesicles that is not completely released during exocytosis (Mellander et al. 2012). Dopamine release could also be controlled by autoregulatory feedback. Drosophila D2 receptors function as presynaptic autoreceptors to regulates dopamine release (Vickrey and Venton 2011). However, studies in anesthetized mice have indicated that autoinhibition by D2 receptors did not have an effect at timescales over 800 ms (Benoit-Marand et al. 2001). Thus, at the timescale of the intervals of the present study, the effect of autoinibibiton would likely be negligible.
The control of dopamine release by synthesis and uptake in Drosophila is similar to mammalian models. Dopamine transporter (DAT) knockout mice have confirmed the importance of DAT in regulating the releasable pool (Jones et al. 1998). In wild-type mice striatal brain slices, dopamine release elicited by a single stimulation pulse does not change with a stimulation interval of 5 min, but release progressively decreases with stimulation intervals of 3 min or less (Kile et al. 2010). The 5 min time scale is consistent with our study in Drosophila. In the caudate nucleus of anesthetized rats, when long (10 s) stimulations are performed, a stimulation interval of approximately 20 min is required to achieve a reproducible response, but the dopamine release is 80 % of the initial stimulation by 5 min (Michael et al. 1987a). In anesthetized rats, the inhibition of synthesis affects stimulations performed 10 min apart, but not when they are less than 2 min apart, and the recycling of dopamine via uptake contributes to the short-term recovery of the releasable pool (Michael et al. 1987a, Michael et al. 1987b). Thus, the timescale of synthesis and uptake to maintain the releasable dopamine pool in Drosophila is similar to that in mammals.
The role of synthesis and uptake in maintaining the releasable serotonin pool has also been investigated in Drosophila (Borue et al. 2010). Uptake is more important for the short time scale and synthesis on the longer time scale for serotonin as well as dopamine. However, dopamine release is independent of synthesis on the 1 min timescale while synthesis plays a role in the replenishment of serotonin release at 1 min intervals (Borue et al. 2010). This discrepancy suggests that synthesis functions on a different time scale for dopamine and serotonin signaling in Drosophila.
There is no cocaine-activated reserve dopamine pool in Drosophila with ATP/P2X2 stimulations
In rodents, cocaine can elevate dopamine release by mobilizing a synapsin-dependent reserve pool of dopamine-containing vesicles (Venton et al. 2006, Kile et al. 2010). Synapsins are phosphoproteins that bind to the cytosolic surface of synaptic vesicles and are important regulators of synaptic transmission. Biochemical studies suggest that the balance between the readily releasable and the reserve pool of synaptic vesicles is regulated phosphorylation of synapsins, as synapsin phosphorylation uncages vesicles and initiates vesicle mobilization (Murthy 2001, Jovanovic et al. 2001, Cousin et al. 2003, Chi et al. 2003). Cocaine is hypothesized to facilitate dopamine release by increasing presynaptic Ca2+ influx, triggering release of the reserve pool as a result of Ca2+-dependent phosphorylation of synapsins (Venton et al. 2006, Kile et al. 2010). One synapsin gene is found in the genome of Drosophila (Klagges et al. 1996), and evidence shows the synapsin mediates mobilization of the reserve pool during intense stimulations (Akbergenova and Bykhovskaia 2007). Studies have identified two functionally and topographically distinct pools of synaptic vesicles in the boutons of the Drosophila larval neuromuscular junction (Kuromi and Kidokoro 1998, Akbergenova and Bykhovskaia 2007, Kuromi and Kidokoro 2002, Michels et al. 2005). However, no reserve pool has been reported in the dopaminergic neurons in Drosophila.
We investigated if cocaine could active a reserve dopamine pool in dopaminergic neurons by inhibiting synthesis and performing repeated stimulations to deplete the releasable pool. Our results showed the dopamine release after cocaine application was not significantly different from that in the control group. One difference between our studies and the rodent studies is the stimulation type: P2X2 stimulations to open ion channels in Drosophila while electrical stimulations were used in rodent models. Drosophila are too small to use traditional electrical stimulation methods. While there is no evidence that cocaine affects P2X2 channels, optogenetic stimulations, such as Channelrhodopsin2 (Vickrey et al. 2009), could be performed in the future to check these results. While the possibility of a reserve dopamine pool activated by other conditions cannot be ruled out, our study indicates that cocaine does not activate the dopamine reserve pool in Drosophila larval VNC with ligand-gated channel stimulation. Thus, there may be a discrepancy in the action of cocaine on reserve pools between Drosophila and mammals and this should be taken into consideration when using Drosophila as a model organism for the study of cocaine addiction (Sovik and Barron 2013, Kaun et al. 2012, Wolf 1999).
In Drosophila, cocaine has a higher affinity for the serotonin transporter than the dopamine transporter (Porzgen et al. 2001). Studies of serotonin regulation in Drosophila found that cocaine does not activate a large serotonin reserve pool, but immunohistochemistry indicates that not all serotonin content in the serotonergic neurons is available for release (Borue et al. 2010). Thus, further study with immunohistochemical staining of nerve cords after depletion of the readily releasable pool could help to identify if reserve dopamine pool exists in Drosophila.
Conclusions
We have characterized ATP/P2X2-mediated dopamine release in Drosophila larval ventral nerve cord and shown that ATP/P2X2-mediated stimulation can be used as a substitute for light-activated channels. Two sources for releasable dopamine pool are identified: newly synthesized dopamine and dopamine recycled via uptake. These two sources act on a different time scale with uptake responsible for rapid replenishment of the releasable pool and synthesis critical for longer-term maintenance of the releasable pool. The role and timescale of synthesis and uptake on the regulation of dopamine signaling in Drosophila is analogous to mammals. However, unlike in mammals, cocaine did not activate a reserve pool in the dopaminergic neurons with ATP/P2X2 stimulation. Our study provides a better understanding of dopamine regulation in Drosophila, thus facilitating the use of this model organism for the study of dopaminergic diseases.
Supplementary Material
Acknowledgments
This work was funded by NIH R01MH085159.
Footnotes
The authors have no conflicts of interest to declare.
References
- Akbergenova Y, Bykhovskaia M. Synapsin maintains the reserve vesicle pool and spatial segregation of the recycling pool in Drosophila presynaptic boutons. Brain Res. 2007;1178:52–64. doi: 10.1016/j.brainres.2007.08.042. [DOI] [PubMed] [Google Scholar]
- Benoit-Marand M, Borrelli E, Gonon F. Inhibition of dopamine release via presynaptic D2 receptors: time course and functional characteristics in vivo. J Neurosci. 2001;21:9134–9141. doi: 10.1523/JNEUROSCI.21-23-09134.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bier E. Drosophila, the golden bug, emerges as a tool for human genetics. Nat Rev Genet. 2005;6:9–23. doi: 10.1038/nrg1503. [DOI] [PubMed] [Google Scholar]
- Borue X, Condron B, Venton BJ. Both synthesis and reuptake are critical for replenishing the releasable serotonin pool in Drosophila. J Neurochem. 2010;113:188–199. doi: 10.1111/j.1471-4159.2010.06588.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brake AJ, Wagenbach MJ, Julius D. New structural motif for ligand-gated ion channels defined by an ionotropic ATP receptor. Nature. 1994;371:519–523. doi: 10.1038/371519a0. [DOI] [PubMed] [Google Scholar]
- Chi P, Greengard P, Ryan TA. Synaptic vesicle mobilization is regulated by distinct synapsin I phosphorylation pathways at different frequencies. Neuron. 2003;38:69–78. doi: 10.1016/s0896-6273(03)00151-x. [DOI] [PubMed] [Google Scholar]
- Collo G, North RA, Kawashima E, Merlo-Pich E, Neidhart S, Surprenant A, Buell G. Cloning OF P2X5 and P2X6 receptors and the distribution and properties of an extended family of ATP-gated ion channels. J Neurosci. 1996;16:2495–2507. doi: 10.1523/JNEUROSCI.16-08-02495.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousin MA, Malladi CS, Tan TC, Raymond CR, Smillie KJ, Robinson PJ. Synapsin I-associated phosphatidylinositol 3-kinase mediates synaptic vesicle delivery to the readily releasable pool. J Biol Chem. 2003;278:29065–29071. doi: 10.1074/jbc.M302386200. [DOI] [PubMed] [Google Scholar]
- Ding S, Sachs F. Single channel properties of P2X2 purinoceptors. J Gen Physiol. 1999;113:695–720. doi: 10.1085/jgp.113.5.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace AA, Floresco SB, Goto Y, Lodge DJ. Regulation of firing of dopaminergic neurons and control of goal-directed behaviors. Trends Neurosci. 2007;30:220–227. doi: 10.1016/j.tins.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Greco PG, Garris PA. In vivo interaction of cocaine with the dopamine transporter as measured by voltammetry. Eur J Pharmacol. 2003;479:117–125. doi: 10.1016/j.ejphar.2003.08.062. [DOI] [PubMed] [Google Scholar]
- Hu A, Zhang W, Wang Z. Functional feedback from mushroom bodies to antennal lobes in the Drosophila olfactory pathway. Proc Natl Acad Sci U S A. 2010;107:10262–10267. doi: 10.1073/pnas.0914912107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Zhang W, Qiao W, Hu A, Wang Z. Functional connectivity and selective odor responses of excitatory local interneurons in Drosophila antennal lobe. Neuron. 2010;67:1021–1033. doi: 10.1016/j.neuron.2010.08.025. [DOI] [PubMed] [Google Scholar]
- Jones SR, Gainetdinov RR, Jaber M, Giros B, Wightman RM, Caron MG. Profound neuronal plasticity in response to inactivation of the dopamine transporter. Proc Natl Acad Sci U S A. 1998;95:4029–4034. doi: 10.1073/pnas.95.7.4029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic JN, Sihra TS, Nairn AC, Hemmings HC, Jr, Greengard P, Czernik AJ. Opposing changes in phosphorylation of specific sites in synapsin I during Ca2+-dependent glutamate release in isolated nerve terminals. J Neurosci. 2001;21:7944–7953. doi: 10.1523/JNEUROSCI.21-20-07944.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaun KR, Devineni AV, Heberlein U. Drosophila melanogaster as a model to study drug addiction. Hum Genet. 2012;131:959–975. doi: 10.1007/s00439-012-1146-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kile BM, Guillot TS, Venton BJ, Wetsel WC, Augustine GJ, Wightman RM. Synapsins differentially control dopamine and serotonin release. J Neurosci. 2010;30:9762–9770. doi: 10.1523/JNEUROSCI.2071-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klagges BR, Heimbeck G, Godenschwege TA, Hofbauer A, Pflugfelder GO, Reifegerste R, Reisch D, Schaupp M, Buchner S, Buchner E. Invertebrate synapsins: a single gene codes for several isoforms in Drosophila. J Neurosci. 1996;16:3154–3165. doi: 10.1523/JNEUROSCI.16-10-03154.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuromi H, Kidokoro Y. Two distinct pools of synaptic vesicles in single presynaptic boutons in a temperature-sensitive Drosophila mutant, shibire. Neuron. 1998;20:917–925. doi: 10.1016/s0896-6273(00)80473-0. [DOI] [PubMed] [Google Scholar]
- Kuromi H, Kidokoro Y. Selective replenishment of two vesicle pools depends on the source of Ca2+ at the Drosophila synapse. Neuron. 2002;35:333–343. doi: 10.1016/s0896-6273(02)00777-8. [DOI] [PubMed] [Google Scholar]
- Lee TH, Balu R, Davidson C, Ellinwood EH. Differential time-course profiles of dopamine release and uptake changes induced by three dopamine uptake inhibitors. Synapse. 2001;41:301–310. doi: 10.1002/syn.1087. [DOI] [PubMed] [Google Scholar]
- Lima SQ, Miesenbock G. Remote control of behavior through genetically targeted photostimulation of neurons. Cell. 2005;121:141–152. doi: 10.1016/j.cell.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Littleton JT, Ganetzky B. Ion channels and synaptic organization: analysis of the Drosophila genome. Neuron. 2000;26:35–43. doi: 10.1016/s0896-6273(00)81135-6. [DOI] [PubMed] [Google Scholar]
- Makos MA, Kim YC, Han KA, Heien ML, Ewing AG. In vivo electrochemical measurements of exogenously applied dopamine in Drosophila melanogaster. Anal Chem. 2009;81:1848–1854. doi: 10.1021/ac802297b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellander LJ, Trouillon R, Svensson MI, Ewing AG. Amperometric post spike feet reveal most exocytosis is via extended kiss-and-run fusion. Sci Rep. 2012;2:907. doi: 10.1038/srep00907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael AC, Ikeda M, Justice JB., Jr Dynamics of the recovery of releasable dopamine following electrical stimulation of the medial forebrain bundle. Neurosci Lett. 1987a;76:81–86. doi: 10.1016/0304-3940(87)90196-0. [DOI] [PubMed] [Google Scholar]
- Michael AC, Ikeda M, Justice JB., Jr Mechanisms contributing to the recovery of striatal releasable dopamine following MFB stimulation. Brain Res. 1987b;421:325–335. doi: 10.1016/0006-8993(87)91302-3. [DOI] [PubMed] [Google Scholar]
- Michels B, Diegelmann S, Tanimoto H, Schwenkert I, Buchner E, Gerber B. A role for Synapsin in associative learning: the Drosophila larva as a study case. Learn Mem. 2005;12:224–231. doi: 10.1101/lm.92805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy VN. Spreading synapsins. Nat Neurosci. 2001;4:1155–1157. doi: 10.1038/nn1201-1155. [DOI] [PubMed] [Google Scholar]
- Neckameyer WS. Multiple roles for dopamine in Drosophila development. Dev Biol. 1996;176:209–219. doi: 10.1006/dbio.1996.0128. [DOI] [PubMed] [Google Scholar]
- Nichols CD. Drosophila melanogaster neurobiology, neuropharmacology, and how the fly can inform central nervous system drug discovery. Pharmacol Ther. 2006;112:677–700. doi: 10.1016/j.pharmthera.2006.05.012. [DOI] [PubMed] [Google Scholar]
- North RA. Molecular physiology of P2X receptors. Physiol Rev. 2002;82:1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- Porzgen P, Park SK, Hirsh J, Sonders MS, Amara SG. The antidepressant-sensitive dopamine transporter in Drosophila melanogaster: a primordial carrier for catecholamines. Mol Pharmacol. 2001;59:83–95. doi: 10.1124/mol.59.1.83. [DOI] [PubMed] [Google Scholar]
- Ross AE, Venton BJ. Nafion-CNT coated carbon-fiber microelectrodes for enhanced detection of adenosine. Analyst. 2012;137:3045–3051. doi: 10.1039/c2an35297d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. Multiple dopamine functions at different time courses. Annu Rev Neurosci. 2007;30:259–288. doi: 10.1146/annurev.neuro.28.061604.135722. [DOI] [PubMed] [Google Scholar]
- Sovik E, Barron AB. Invertebrate models in addiction research. Brain Behav Evol. 2013;82:153–165. doi: 10.1159/000355506. [DOI] [PubMed] [Google Scholar]
- Stamford JA, Kruk ZL, Millar J. Dissociation of the actions of uptake blockers upon dopamine overflow and uptake in the rat nucleus accumbens: in vivo voltammetric data. Neuropharmacology. 1989;28:1383–1388. doi: 10.1016/0028-3908(89)90014-2. [DOI] [PubMed] [Google Scholar]
- Swamy BE, Venton BJ. Carbon nanotube-modified microelectrodes for simultaneous detection of dopamine and serotonin in vivo. Analyst. 2007;132:876–884. doi: 10.1039/b705552h. [DOI] [PubMed] [Google Scholar]
- Venken KJ, Simpson JH, Bellen HJ. Genetic manipulation of genes and cells in the nervous system of the fruit fly. Neuron. 2011;72:202–230. doi: 10.1016/j.neuron.2011.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venton BJ, Seipel AT, Phillips PE, Wetsel WC, Gitler D, Greengard P, Augustine GJ, Wightman RM. Cocaine increases dopamine release by mobilization of a synapsin-dependent reserve pool. J Neurosci. 2006;26:3206–3209. doi: 10.1523/JNEUROSCI.4901-04.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venton BJ, Wightman RM. Psychoanalytical electrochemistry: Dopamine and behavior. Anal Chem. 2003;75:414A–421A. [Google Scholar]
- Vickrey TL, Condron B, Venton BJ. Detection of endogenous dopamine changes in Drosophila melanogaster using fast-scan cyclic voltammetry. Anal Chem. 2009;81:9306–9313. doi: 10.1021/ac901638z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickrey TL, Venton BJ. Drosophila Dopamine2-like receptors function as autoreceptors. ACS Chem Neurosci. 2011;2:723–729. doi: 10.1021/cn200057k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickrey TL, Xiao N, Venton BJ. Kinetics of the dopamine transporter in Drosophila larva. ACS Chem Neurosci. 2013;4:832–837. doi: 10.1021/cn400019q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf ME. Cocaine addiction: clues from Drosophila on drugs. Curr Biol. 1999;9:R770–R772. doi: 10.1016/S0960-9822(00)80009-3. [DOI] [PubMed] [Google Scholar]
- Xiao N, Privman E, Venton BJ. Optogenetic control of serotonin and dopamine release in Drosophila larvae. ACS Chem Neurosci. 2014;5:666–673. doi: 10.1021/cn500044b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Z, Macara AM, Lelito KR, Minosyan TY, Shafer OT. Analysis of functional neuronal connectivity in the Drosophila brain. J Neurophysiol. 2012;108:684–696. doi: 10.1152/jn.00110.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


