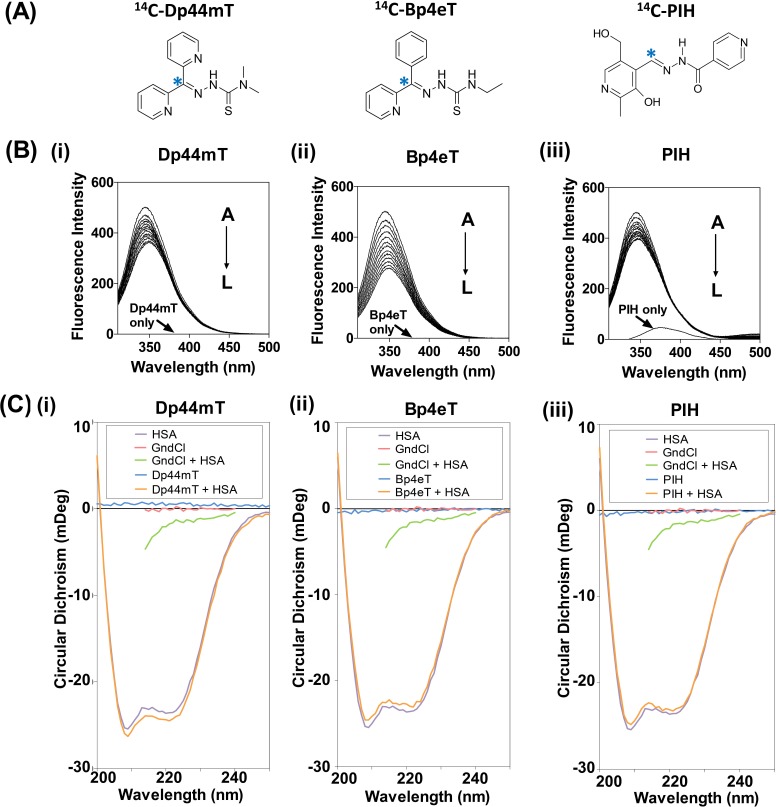
Figure 1. (A): Line drawings of the chemical structures of the iron chelators: di-2-pyridylketone 4,4-dimethyl-3-thiosemicarbazone (Dp44mT), 2-benzoylpyridine 4-ethyl-3-thiosemicarbazone (Bp4eT) and pyridoxal isonicotinoyl hydrazone (PIH).
Asterisk (*) indicates position of the 14C-label. (B) Fluorescence emission spectrum of HSA (2 μM) excited at 295 nm in the presence of increasing concentrations (A→L; 0-3.67 μM) of: (i) Dp44mT; (ii) Bp4eT; or (iii) PIH in PBS at 37°C/pH 7.4. (C) Circular dichroism of HSA (2 μM) in the presence of: (i) Dp44mT, (ii) Bp4eT or (iii) PIH (10 μM) after a 2 h incubation at 37°C. Results shown are typical of 3 experiments performed.