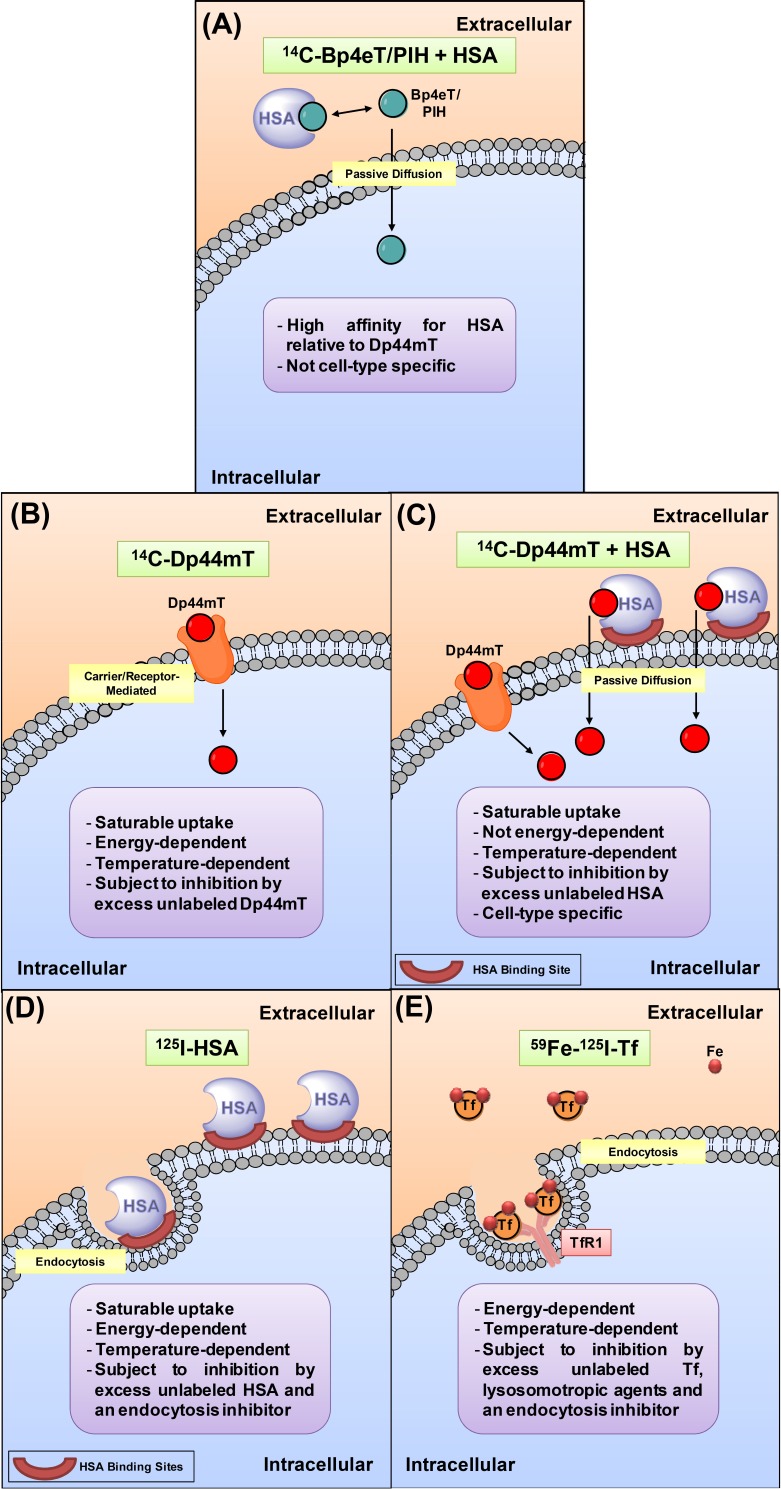
Figure 7. Schematic showing the internalization of 14C-Bp4eT/PIH or 14C-Dp44mT with or without HSA, 125I-HSA or 59Fe-125I-Tf into the cell.
(A) 14C-Bp4eT and 14C-PIH are transported by diffusion in the absence of HSA [34]. In the presence of HSA, HSA-binding inhibits the uptake of 14C-Bp4eT and 14C-PIH, irrespective of the cell-type. This is due to the high affinity of Bp4eT or PIH for HSA, relative to Dp44mT, reducing the levels of free drug available to diffuse into cells. (B) A different mechanism is demonstrated by the structurally similar ligand, Dp44mT. 14C-Dp44mT is taken up by cells via a receptor/carrier in the absence of HSA. This uptake process is saturable, energy- and temperature-dependent and subject to inhibition by excess unlabeled Dp44mT [34]. (C) In the presence of HSA, 14C-Dp44mT uptake occurs through a second, high capacity, saturable process that is cell-type specific and inhibited in the presence of excess unlabeled HSA. This process may be facilitated by: (i) a specific HSA-binding site; (ii) the fact most cellular HSA is bound to the cell membrane (rather than internalized); and (iii) the relatively low affinity of Dp44mT for HSA. These three properties facilitate Dp44mT delivery to cells and create a concentration gradient at the cell surface to enable enhanced uptake via dissociation and passive diffusion. The enhanced delivery of 14C-Dp44mT by HSA increases apoptosis and cytotoxicity. (D) 125I-HSA uptake by cells was saturable, temperature-dependent, inhibited by excess unlabeled HSA, and sensitive to glucose starvation and inhibitors of energy metabolism or endocytosis. Hence, this process was consistent with HSA endocytosis that is known to occur [74, 92]. (E) 59Fe-125I-Tf uptake occurs following the binding to its receptor, Tf receptor 1 (TfR1). This process was temperature- and energy-dependent, and subject to inhibition by excess unlabeled Tf, an endocytosis inhibitor, and in addition, lysosomotropic agents. Hence, 59Fe-125I-Tf uptake occurs through the well characterized process of receptor-mediated endocytosis that requires endosomal acidification [52, 68, 71, 93].