Infection of a host results in the activation, proliferation and differentiation of rare naive antigen-specific CD8+ T cells. Appropriate initiation of the CD8+ T-cell response occurs when the lymphocyte interacts with its cognate antigen presented by MHC class I on antigen-presenting cells within secondary lymphoid organs. During this process, the antigen-presenting cells actively shape the early CD8+ T-cell response by their expression of costimulatory molecules and secretion of cytokines. This results in the exponential expansion of antigen-specific CD8+ T cells. Subsequently, the majority of the antigen-specific CD8+ T cells die during contraction, while a small proportion of the antigen-specific CD8+ T cells survive to form the memory CD8+ T-cell population. Numerous studies have shown that the effector CD8+ T-cell population found at the peak of the immune response is extremely heterogeneous with each subpopulation having different memory potential.1 Heterogeneity within the effector CD8+ T-cell population can be resolved by a combination of IL-7Rα/CD127 and KLRG1 expression to distinguish terminal effector CD8+ T cells with a shorter half-live (IL-7Rα/CD127low KLRG1high; SLECs) or effector CD8+ T cells with a longer half-life and greater memory potential (IL-7Rα/CD127high KLRG1low; MPECs).2 Further heterogeneity within the MPEC population can be revealed by CD62L and CD103 expression to identify CD62Lhigh central-memory precursors (Tcm MPECs), CD62Llow effector-memory precursors (Tem MPECs) and CD103high tissue-resident memory cells (Trm) MPECs.3,4 However, it has been thought that each effector CD8+ T-cell subpopulation passes through a requisite cytotoxic effector stage.5 In this issue of Cellular and Molecular Immunology, Sarkar and colleagues provide further in vivo evidence that each effector CD8+ T-cell subpopulation, regardless of memory potential, passes through a requisite stage of high cytotoxic (CTL) activity that is tuned by the local immune microenvironment.6
In vivo antibody staining for analysis of the immune response
Our understanding of the CD8+ T-cell response was been highly dependent on ex vivo analysis of responding CD8+ T cells in order to understand their biology. Slowly, we have increased the arsenal of in vivo assays to assess their activation, differentiation, functionality and localization. Importantly, these assays have both confirmed and challenged what the field has found using ex vivo assays. In this issue of Cellular and Molecular Immunology, Sarkar and colleagues6 further enhance our ability to analyze cellular populations in vivo by developing a staining method to track both the in vivo phenotype and CTL effector function of responding CD8+ T cells. To stain CD8+ T cells in vivo, Sarkar and colleagues6 administered monoclonal antibodies intravenously for 1–2 h prior to tissue collection allowing the diffusion of the monoclonal antibodies into the tissue to effectively stain the entire CD8+ T-cell population.6 This staining protocol recapitulated the phenotype of effector CD8+ T cells observed during the acute phase of lymphocytic choriomeningitis virus (LCMV) using standard ex vivo analysis. Yuzefpolskiy et al. couple this traditional in vivo staining assay with in vivo cell surface expression of CD107a/b,6 which marks lytic granule release (degranulation) within T-cell populations. Using this novel in vivo degranulation assay to assess CTL effector function, Yuzefpolskiy et al.6 confirm that early after infection both SLECs and MPECs have equivalent effector function and transition through a requisite CTL effector phase in vivo, consistent with previous ex vivo markers of effector function.7 Excitingly, Sarkar and colleagues'6 in vivo degranulation assay also contributes important new information to the field. First, they found that the CTL effector program was rapidly shut down within the lymph nodes.6 Second, at later time points (8 d.p.i.) after acute LCMV infection, the KLRG-1highCD8+ T cells (SLECs) had decreased CTL effector activity suggesting that SLECs in an antigen rich tissue may be more likely to undergo functional exhaustion and cell death.6 Thus, this work highlights that understanding the spatial location of effector CD8+ T cells has important implications in understanding effector versus memory differentiation.8,9
Insights into effector and memory CD8+ T-cell differentiation pathways
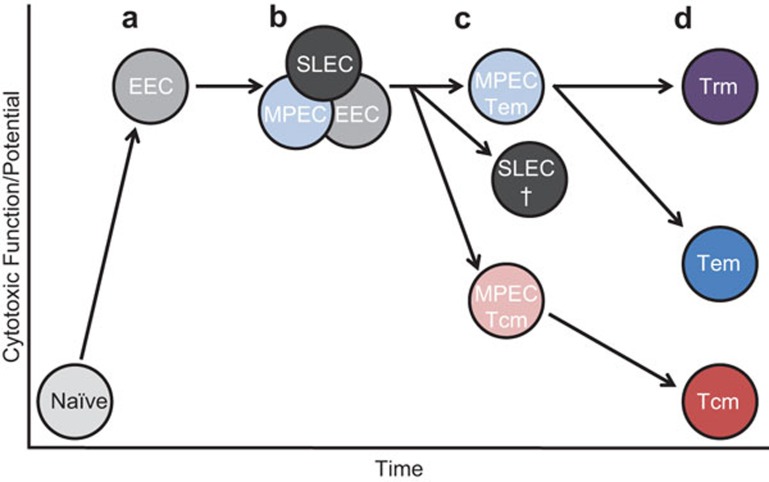
This work by Sarkar and colleagues6 provides further support for the linear differentiation model of effector and memory CD8+ T-cell differentiation.5 Upon activation, rare naive antigen-specific CD8+ T cells undergo robust proliferation, differentiation and acquisition and retention of effector function (Figure 1). The first effector population observed is a IL-7Rα/CD127low KLRG1lowearly effector cell (EEC) population (Figure 1a).7 Based on secondary environmental signals received by these initial EECs subsequent growth and/or differentiation of those cells into EECs, SLECs and MPECs may occur.1,2,7 As shown here by Yuzefpolskiy et al.,6 these early effector CD8+ T cells (4.5 d.p.i.) have high in vivo degranulation,6 which correlated well with ex vivo analysis for granzyme B in these populations (Figure 1b).7 By the peak of the CD8+ T-cell response, Yuzefpolskiy et al.6 found that effector CD8+ T cells in the lymph nodes displayed reduced in vivo degranulation, consistent with the ex vivo observation that granzyme B levels within the central memory (Tcm) MPEC population was more rapidly downregulated (Figure 1c).7 Moreover, the fact that lymph node-derived effector CD8+ T cells shut down their CTL effector program earlier than other tissues might indicate that those cells undergo fewer antigen encounters and/or an inflammatory environment more conducive with memory cell differentiation, thus explaining why effector CD8+ T cells from lymph nodes were the best source of memory precursors irrespective of their cell surface phenotype.9 However, in peripheral organs that are more rich in viral antigen, Yuzefpolskiy et al.6 found that MPECs had enhanced in vivo degranulation over SLECs (Figure 1c). This seemingly counterintuitive finding could shed some light on in vivo CD8+ T-cell biology. First, this result suggests that SLECs may undergo continual engagement with cognate antigen which eventually leads to their functional exhaustion and death. Second, the MPECs may be recently recruited to or preferentially survive within peripheral tissues and sites of antigen occurrence to maintain an effective, sterilizing immune response. Furthermore, retention of effector function by MPECs in peripheral tissues might explain why Trm and Tem populations retain high effector function potential at memory.4,10
Figure 1.
Potential pathway for the development of effector and memory CD8+ T-cell populations in relation to their effector function. Upon activation naive antigen-specific CD8+ T cells rapidly downregulate the IL-7 receptor to form an EEC population. This population is the earliest effector phase that can be distinguished based on KLRG1 and IL-7R and is already associated with high levels of CTL function and potential (a). EECs may further differentiate into short-lived, terminally differentiated SLECs or long-lived memory precursors (MPECs), or remain phenotypically as an EEC. These effector phases are also associated with high levels of CTL effector function, as antigen is present and granzyme B expression can be linked to in vivo and ex vivo degranulation (b). As effector T-cell populations contract and memory populations are established, a hierarchy of CTL effector potential emerges from these populations (c, d). Trm residing in peripheral tissues express constitutively high granzyme B levels, whereas Tem and Tcm display a reduced CTL potential, at least until antigen re-exposure.
Conclusions
This exciting new in vivo degranulation assay developed by Sarkar and colleagues6 further solidifies the notion that all CD8+ T cells pass through a requisite cytotoxic stage, and also elucidates novel biology occurring during an in vivo CD8+ T-cell response. Understanding how migratory, survival or retention patterns of effector CD8+ T cells regulate in vivo effector function, generation of protective immunity and long-lived memory CD8+ T-cell formation using this new in vivo degranulation assay and other newly developed techniques provides an exciting future for T-cell biology.
Acknowledgments
JJO is supported by the Montana Agricultural Experiment State. Both JJO and BSS are supported by grants from the National Institutes of Health.
References
- Kaech SM, Cui W. Transcriptional control of effector and memory CD8+ T cell differentiation. Nat Rev Immunol. 2012;12:749–761. doi: 10.1038/nri3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi NS, Kaech SM. Effector CD8 T cell development: a balancing act between memory cell potential and terminal differentiation. J Immunol. 2008;180:1309–1315. doi: 10.4049/jimmunol.180.3.1309. [DOI] [PubMed] [Google Scholar]
- Obar JJ, Lefrancois L. Early signals during CD8 T cell priming regulate the generation of central memory cells. J Immunol. 2010;185:263–272. doi: 10.4049/jimmunol.1000492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan BS, Pham QM, Lee YT, Cauley LS, Puddington L, Lefrançois L. Oral infection drives a distinct population of intestinal resident memory CD8+ T cells with enhanced protective function. Immunity. 2014;40:747–757. doi: 10.1016/j.immuni.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefrancois L, Masopust D. The road not taken: memory T cell fate ‘decisions'. Nat Immunol. 2009;10:369–370. doi: 10.1038/ni0409-369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuzefpolskiy Y, Baumann F, Kalia V, Sarkar S. Early CD8 T cell memory precursors exhibit potent in vivo degranulation. Cell Mol Immunol. 2014;12:400–408. doi: 10.1038/cmi.2014.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obar JJ, Jellison ER, Sheridan BS, Blair DA, Pham QM, Zickovich JM, et al. Pathogen-induced inflammatory environment controls effector and memory CD8+ T cell differentiation. J Immunol. 2011;187:4967–4978. doi: 10.4049/jimmunol.1102335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung YW, Rutishauser RL, Joshi NS, Haberman AM, Kaech SM. Differential localization of effector and memory CD8 T cell subsets in lymphoid organs during acute viral infection. J Immunol. 2010;185:5315–5325. doi: 10.4049/jimmunol.1001948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedzierska K, Stambas J, Jenkins MR, Keating R, Turner SJ, Doherty PC. Location rather than CD62L phenotype is critical in the early establishment of influenza-specific CD8+ T cell memory. Proc Natl Acad Sci USA. 2007;104:9782–9787. doi: 10.1073/pnas.0703699104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masopust D, Vezys V, Marzo AL, Lefrancois L. Preferential localization of effector memory cells in nonlymphoid tissue. Science. 2001;291:2413–2417. doi: 10.1126/science.1058867. [DOI] [PubMed] [Google Scholar]