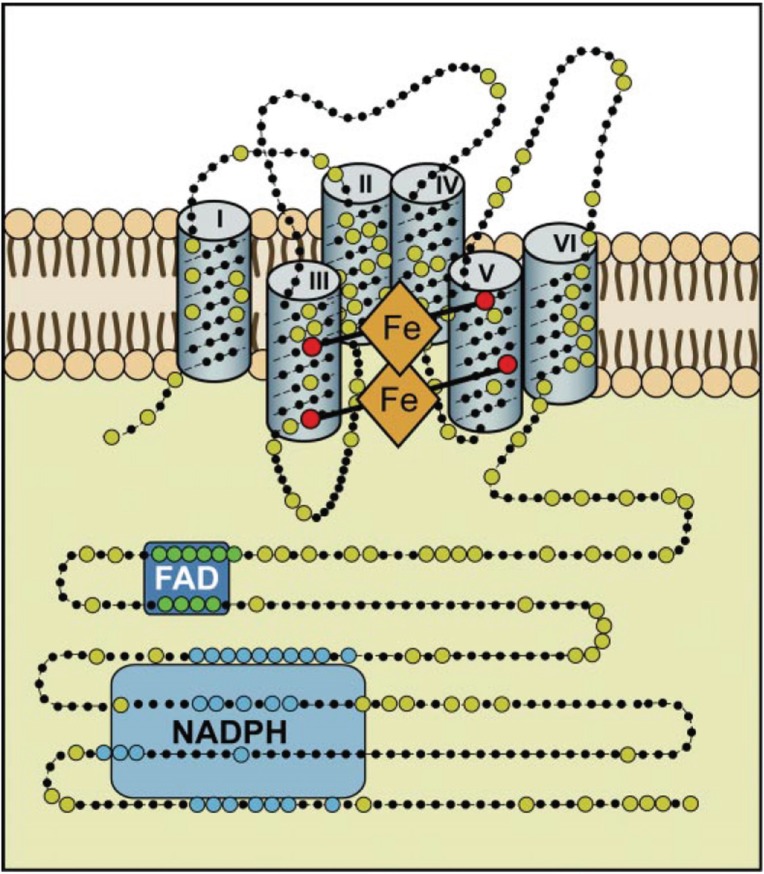
Figure 3.
Hypothetical structure of a typical NADPH oxidase. This structural model is based on bioinformatics, cell fractionation and biochemical data concerning the human Nox enyzmes (NOX1, 2, 3, and 4); no crystallographic or NMR structural data are available yet. Nox enzymes comprise typically about 500 amino acids and are exclusively located in lipid bylayer membranes, like the plasma membrane or the ER membrane. Large dots are highly conserved amino acids. The reaction center transferring a single electron to oxygen is the upper b-type heme in this scheme. The enzyme consists of six transmembrane helices. The two b-type hemes are coordinated with histidine residues between helices III and V. The enzyme contains binding sequences for NADPH as well as for FAD in its cytoplasmic tail (after Bedard and Krause 2007 [60]).