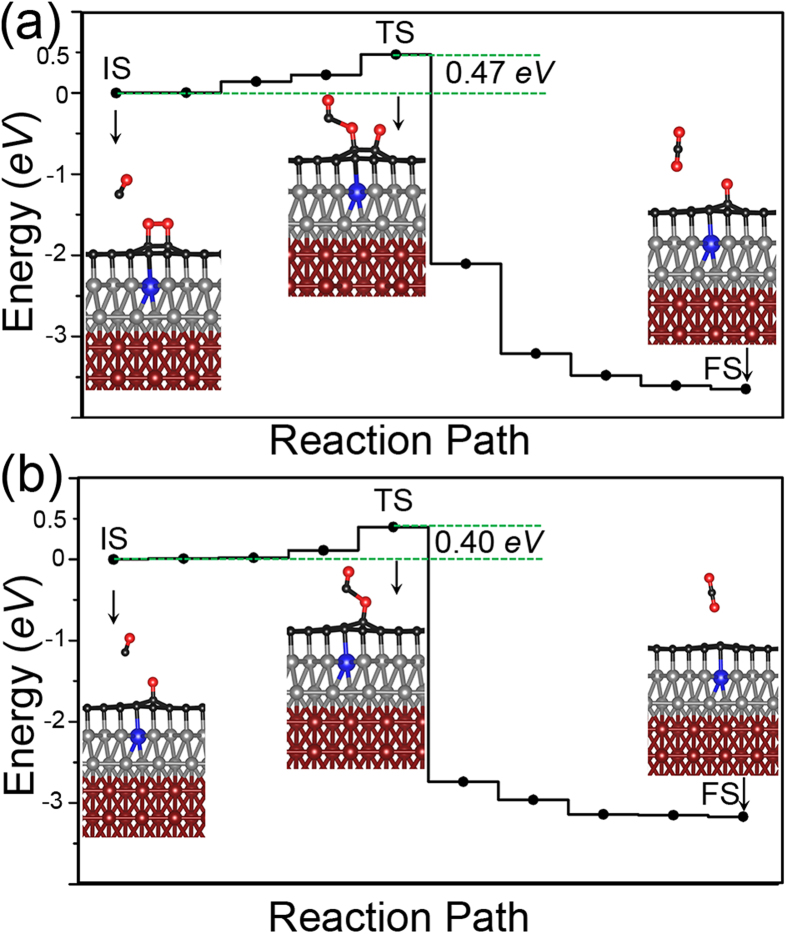
Figure 5. The energy profile of two-step CO oxidation catalyzed by graphene supported on Zn@Fe/Ni (111).
The calculations are done by the c-NEB method. (a) the first step of the reaction: CO+O2→CO2+O. The initial state (IS): d(O-O) = 1.48 Å, d(C-O) = 1.14 Å, and d(CO-O2) = 3.10 Å; The transition state (TS): d(O-O) = 2.12 Å, d(C-O) = 1.16 Å, and d(CO-O2)=1.85 Å, in the final state (FS), CO2 forms. (b) the second step of the reaction: CO+O→CO2. The initial state (IS): d(C-O)=1.14 Å, and d(CO-O2) = 3.30 Å; The transition state (TS): d(C-O) = 1.17 Å, and d(CO-O) = 1.76 Å. CO2 does not bind to the graphene surface in both cases. Note that d(O-O) denotes the O-O bond length of O2, d(C-O) the C-O bond length of CO, and d(CO-O2) the distance between the C atom in CO and the nearest O in O2.