Abstract
Objective To follow up on previously reported associations between periconceptional use of selective serotonin reuptake inhibitors (SSRIs) and specific birth defects using an expanded dataset from the National Birth Defects Prevention Study.
Design Bayesian analysis combining results from independent published analyses with data from a multicenter population based case-control study of birth defects.
Setting 10 centers in the United States.
Participants 17 952 mothers of infants with birth defects and 9857 mothers of infants without birth defects, identified through birth certificates or birth hospitals, with estimated dates of delivery between 1997 and 2009.
Exposures Citalopram, escitalopram, fluoxetine, paroxetine, or sertraline use in the month before through the third month of pregnancy. Posterior odds ratio estimates were adjusted to account for maternal race/ethnicity, education, smoking, and prepregnancy obesity.
Main outcome measure 14 birth defects categories that had associations with SSRIs reported in the literature.
Results Sertraline was the most commonly reported SSRI, but none of the five previously reported birth defects associations with sertraline was confirmed. For nine previously reported associations between maternal SSRI use and birth defect in infants, findings were consistent with no association. High posterior odds ratios excluding the null value were observed for five birth defects with paroxetine (anencephaly 3.2, 95% credible interval 1.6 to 6.2; atrial septal defects 1.8, 1.1 to 3.0; right ventricular outflow tract obstruction defects 2.4, 1.4 to 3.9; gastroschisis 2.5, 1.2 to 4.8; and omphalocele 3.5, 1.3 to 8.0) and for two defects with fluoxetine (right ventricular outflow tract obstruction defects 2.0, 1.4 to 3.1 and craniosynostosis 1.9, 1.1 to 3.0).
Conclusions These data provide reassuring evidence for some SSRIs but suggest that some birth defects occur 2-3.5 times more frequently among the infants of women treated with paroxetine or fluoxetine early in pregnancy.
Introduction
The association between maternal use of antidepressants, especially selective serotonin reuptake inhibitors (SSRIs), during pregnancy and birth defects in the infants has been the topic of much discussion in recent years. After initial reports of an association between paroxetine and heart defects, the US Food and Drug Administration published an advisory warning of this potential association in December 2005.1 Recent meta-analyses and systematic reviews combining data from more than 20 epidemiological studies have reached conflicting conclusions and this uncertainty influences perceptions of the safety of antidepressant use in pregnancy.2 3 In a recent study, 69% of women thought that it was definitely or probably acceptable to take such drugs when not pregnant or breast feeding, but only 33% of women thought that it was definitely or probably acceptable to do so when pregnant.4 SSRIs are increasingly used by women of reproductive age and during pregnancy, but the inconsistent reports have limited opportunities for clinicians to carefully evaluate the risk compared with benefit of specific SSRIs for a given patient during pregnancy.5 6 7
We reviewed the literature for any reports that assessed the relation between specific SSRIs and one or more of the specific birth defects that are also included in the US National Birth Defects Prevention Study (NBDPS).2 8 9 10 11 12 13 To provide a more robust estimate of the association between individual SSRIs and birth defects, information that is necessary for decision making by patients who are being treated with these drugs and their physicians, we used bayesian methods both to summarize independent findings identified in the literature and to update those findings using the entire set of data from the NBDPS.14
Methods
Study population
For this analysis we used data from the NBDPS, a population based case-control study of birth defects. The study’s methods have been described previously.15 16 17 Briefly, cases of birth defects were identified through birth defects surveillance systems in the US states of Arkansas, California, Georgia, Iowa, Massachusetts, New Jersey, New York, North Carolina, Texas, and Utah. Cases could be live born, stillborn, or induced abortions with one of over 30 major birth defects. The NBDPS excluded cases with known chromosomal or monogenic disorders. Unmatched liveborn controls from the same geographical region and time period were selected from birth certificates or birth hospital records. More cases than controls were included overall because the study was designed for the assessment of individual defects, where controls, which were the same for all case groups, outnumbered even the largest case group. Mothers were asked to participate in a telephone interview in English or Spanish between six weeks and two years after the estimated date of delivery.
For this analysis we included cases and controls if they were born on or after 1 October 1997 and had an estimated date of delivery on or before 31 December 2009. Overall participation was 67.4% for cases and 64.8% for controls. A previous NBDPS analysis of the association between SSRI use during pregnancy and birth defects, included in table 1 as Alwan and colleagues, included data from 1997 to 2002.8 To avoid double counting, these NBDPS data were excluded from the meta-analyses used to calculate prior odds ratios in the current study, but we used significant findings from the previous analysis along with those of the other studies listed in table 1 to determine which birth defect-SSRI combinations to assess. Because of the strong association between diabetes and birth defects, we excluded case and control mothers who reported pregestational diabetes (type 1 or type 2).18 We also excluded mothers who reported the use of any of the following known teratogenic treatments: misoprostol, methotrexate, mycophenolate mofetil, thalidomide, or isotretinoin.19
Table 1.
Previous studies that assessed use of individual selective serotonin reuptake inhibitors (SSRIs) during early pregnancy in mother and individual birth defects in infant
| Authors | Study design | Controls/comparison group | Population | Included birth years | SSRIs | Birth defect categories assessed | Included confounders |
|---|---|---|---|---|---|---|---|
| Alwan et al 20078 | Case-control (subset of current study) | Liveborn infants without major defects | 10 sites in USA | 1997-2002 | Fluoxetine, paroxetine, sertraline | All defects, with at least three exposed | Race/ethnicity, obesity, smoking, income |
| Louik et al 200711 | Case-control | Non-malformed infants | Boston, Philadelphia, Toronto, San Diego, New York State | 1993-2004 | Citalopram, fluoxetine, paroxetine, sertraline | All defects | Maternal age, race/ethnicity, education, year of last menstrual period, study center, smoking, alcohol, family history, body mass index, parity, seizures, diabetes, infertility, hypertension, folic acid use |
| Bakker et al 20109 | Case-control | Fetuses and children with chromosomal or single gene disorder | Population based birth defects registry in Northern Netherlands | 1997-2006 | Paroxetine | Heart defects categories | Year of birth |
| Kornum et al 201010 | Cohort | Children of women without SSRI prescription | Northern Denmark | 1991-2007 | Citalopram, escitalopram, fluoxetine, paroxetine, sertraline | Septal heart defects | Smoking, age, birth order, birth year |
| Reis and Kallen 201013 | Cohort | Children of women without antidepressant use | Sweden | 1995-2007 | Citalopram, fluoxetine, paroxetine, sertraline | Hypospadias | Year of birth, age, parity, smoking and body mass index |
| Malm et al 201112 | Cohort | Children of women without SSRI prescription reimbursements | Finland | 1996-2006 | Citalopram, escitalopram, fluoxetine, paroxetine, sertraline | All defects | Age, parity, year of birth, marital status, smoking, other psychiatric medicines, diabetes |
SSRI use
During the interview for the NBDPS, no specific question addressed depression. Mothers were asked if they had any illnesses other than the ones already discussed (for example, hypertension or diabetes) and whether they took any medication for the illness. Women could report depression here, which would then be followed by a question about any medications taken for the illness. There were also specific medication related questions: “between three months before conception and [the baby’s] date of birth, did you take any of the following medications? Prozac? Paxil? Zoloft? Celexa?” There was no specific question for Lexapro. For this analysis, we considered women exposed if they reported taking citalopram (Celexa), escitalopram (Lexapro), fluoxetine (Prozac), paroxetine (Paxil), or sertraline (Zoloft) at least once in the period from one month before conception through the third month of pregnancy. Women who reported taking more than one type of SSRI were only included in the multiple SSRI category. We considered women as unexposed if they did not take any antidepressants in the period from three months before to the end of the pregnancy and did not report any depression, anxiety, bipolar disorder, or obsessive compulsive disorder. Women who did not answer all medication related questions, used SSRIs in a period other than the period of interest, or took antidepressants other than SSRIs (for example, bupropion and venlafaxine) were excluded.
Birth defects
The NBDPS includes over 30 categories of major birth defects. After ascertainment in population based surveillance systems, the diagnostic information was reviewed by clinical geneticists at each site to establish eligibility. Designated individual clinical geneticists reviewed all cases with a particular defect to ensure consistency across sites.16 Because the primary intent of this analysis was to assess previously reported associations with SSRIs and to determine if those associations were supported by NBDPS data, we included only outcomes with at least one previous report in the peer reviewed literature suggesting a possible association with SSRIs: neural tube defects (international classification of diseases, ninth revision (ICD-9): 740-742.0), anencephaly (ICD-9: 740), all septal defects (ICD-9: 745), ventricular septal defects (ICD-9: 745.4), right ventricular outflow tract obstructions (ICD-9: 746.0-746.1), cleft palate (ICD-9: 749.0), cleft lip with or without cleft palate (ICD-9: 749.2-749.4), esophageal atresia (ICD-9: 750.3), anal atresia (ICD-9: 751.23-751.24), hypospadias (ICD-9: 752.6), any limb reduction defect (ICD-9: 755.2), craniosynostosis (ICD-9: 756.0), gastroschisis (ICD-9: 756.71), and omphalocele (ICD-9: 756.70). Some defects reported in other studies (for example, cystic kidney) could not be evaluated in this analysis because they were not included in NBDPS.13
Development of priors
A bayesian approach requires specification of prior distributions for each of the variables in the model used to estimate the potential association between risk of birth defect and use of SSRIs. These prior distributions are probabilistic summaries of beliefs about the true values of the unknown variables before assessment of new data. The bayesian approach allowed us to incorporate existing information on the association between each SSRI and the birth defect outcome of interest. Prior distributions were developed based on literature review. A systematic review identified six studies published before 2010 that had available specific information on SSRI-birth defect combinations (table 1).8 9 10 11 12 13
We created categories of birth defects based on those reports, which corresponded as closely as possible to the NBDPS birth defect categories (table 1).8 9 10 11 12 13 The approach used to summarize the information presented in these publications for each of the SSRI-birth defect combinations of interest depended on the number of available studies; to avoid duplication of cases in the current analysis, we did not include the results from the earlier NBDPS analysis.8 If one published assessment was only available, then the prior distribution for the log of the odds ratio relating the birth defect and use of SSRIs was assumed to be normal, with a mean given by the log odds ratio estimate reported in the study and variance defined using the corresponding reported confidence interval. If two or more studies were identified, we used bayesian meta-analysis methods to summarize the results. The goal of the meta-analysis was to produce an estimate of the log odds ratio relating the specific birth defect and SSRI across studies, taking into account the study specific estimates and their associated sampling errors. The assumed meta-analysis model included a term corresponding to the true underlying log odds ratio relating SSRI use and risk of birth defects and a collection of random study level effects.20 All available information was included in developing the meta-analysis based prior estimates, including results indicating no association between risk of birth defects and SSRI use from other published studies. If no information other than the previous NBDPS analysis8 was identified, then we assumed the log odds ratio relating birth defects and SSRI use to have a non-informative prior distribution, defined using a normal distribution with mean zero and a variance of 1000. Using this non-informative prior places virtually the entire weight in developing the bayesian estimates on the information contained in the full NBDPS data.
An alternative approach to this analysis would be to develop estimates of the association between SSRI consumption and risk of birth defects using frequentist methods only focused on the 1997-2009 NBDPS data. These results could be summarized and then included as an additional point in a larger meta-analysis of available information. We chose the bayesian approach for two primary reasons. Firstly, we viewed the collection of information summarized by the meta-analysis as the state of current knowledge concerning potential association between SSRIs and risk of birth defects and the NBDPS data as new information available to update that knowledge. This view is consistent with the bayesian updating paradigm as applied in this analysis. In addition, we believe that only utilizing summary values (for example, estimated odds ratios and their standard errors) from the NBDPS data would be an unnecessary sacrifice of information as opposed to utilizing the individual level data informed by the meta-analysis priors.
Bayesian analysis
We used a bayesian approach to develop estimates for a logistic regression model relating the log odds of a specific defect and the mother’s use of SSRIs (see supplementary appendix 1). In addition to a term reflecting the log odds ratio relating birth defects and use of SSRIs, the model also included confounders selected a priori and obtained through the maternal interview: maternal race/ethnicity (non-Hispanic white versus other), maternal education (0-12 years versus >12 years), obesity (body mass index <30 versus ≥30), and smoking (any smoking versus no smoking from one month before to the end of the first trimester). Although prior probabilities for the variable relating birth defect risk and use of SSRIs were developed, we assumed non-informative priors for the odds ratios associated with maternal race/ethnicity, education, obesity, and smoking in the logistic regression model. Posterior estimates for the model variables were developed using Markov Chain Monte Carlo methods. BUGS was used for the bayesian analyses.21
The primary results presented here were derived using an analysis based only on NBDPS participants who reported values for all the variables used in the logistic regression model. We also conducted sensitivity analyses focused on assessing the impact of not including participants with missing information, including consideration of a potential association between being missing and the unknown outcome. This analysis utilized bayesian imputation for missing data both under an assumption that the missing information was missing at random and under plausible assumptions on mechanisms for informative missingness (see supplementary appendix 1).
Results
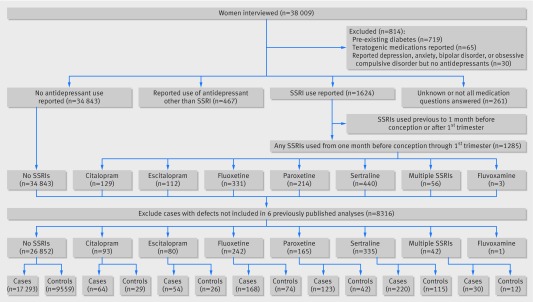
A total of 38 009 women with births between 1997 and 2009 were interviewed for the NBDPS. We excluded women reporting pre-existing diabetes (n=719), known use of teratogenic drugs (n=65), or depression, anxiety, bipolar disorder, or obsessive compulsive disorder but not reporting any antidepressant use (n=30). And, after excluding defects with no previous reported associations with SSRIs, the final analyses included 17 293 unexposed cases, 659 cases exposed to citalopram, escitalopram, fluoxetine, paroxetine or sertraline, 9559 unexposed controls, and 298 controls that were exposed to one of these SSRIs (figure).
Flow chart of participants through study
Sertraline was the most commonly used SSRI; approximately 40% of control mothers who reported use of an SSRI used sertraline (table 2). Although there was some difference in overall SSRI use by state, the distribution of the specific SSRIs was similar across the study sites. There was no difference in the age distribution among SSRI users except for sertraline, which was more often reported among older mothers. Reported use of citalopram and escitalopram started in 2000 and 2002, respectively, and increased over time. Fluoxetine reports decreased over time, but not as much as paroxetine use decreased, and sertraline use stayed relatively constant. There was no difference in the use of the specific SSRIs before or after 1 December 2006, the date the American College of Obstetricians and Gynecologists published their committee opinion on SSRI use in pregnancy.22 23
Table 2.
Descriptive statistics of control mothers (n=9857) who reported periconceptional use of selective serotonin reuptake inhibitors (SSRIs), National Birth Defects Prevention Study, 1997-2009
| Variables | No (%) using SSRIs | ||||||
|---|---|---|---|---|---|---|---|
| Any SSRIs | Citalopram | Escitalopram | Fluoxetine | Paroxetine | Sertraline | Multiple SSRIs | |
| Total | 298 (3.0) | 29 (9.7) | 26 (8.7) | 74 (24.8) | 42 (14.1) | 115 (38.6) | 12 (4.0) |
| Study site*: | |||||||
| New Jersey | 7 (1.2) | 0 | 0 | 1 (14) | 3 (43) | 3 (43) | 0 |
| Texas | 17 (1.4) | 1 (6) | 1 (6) | 3 (18) | 2 (12) | 9 (53) | 1 (6) |
| California | 24 (2.1) | 1 (4) | 2 (8) | 8 (33) | 2 (8) | 8 (33) | 3 (13) |
| Georgia | 23 (2.2) | 3 (13) | 1 (4) | 8 (35) | 1 (4) | 9 (39) | 1 (4) |
| New York | 21 (2.5) | 2 (10) | 1 (5) | 4 (19) | 5 (24) | 8 (38) | 1 (5) |
| Arkansas | 43 (3.5) | 3 (7) | 4 (9) | 11 (26) | 2 (5) | 20 (47) | 3 (7) |
| North Carolina | 28 (3.6) | 2 (7) | 5 (18) | 4 (14) | 5 (18) | 12 (43) | 0 |
| Massachusetts | 42 (3.6) | 5 (12) | 2 (5) | 12 (29) | 7 (17) | 16 (38) | 0 |
| Iowa | 42 (3.9) | 3 (7) | 5 (12) | 9 (21) | 10 (24) | 13 (31) | 2 (5) |
| Utah | 51 (6.1) | 9 (18) | 5 (10) | 14 (27) | 5 (10) | 17 (33) | 1 (2) |
| Maternal age (years): | |||||||
| <20 | 14 (1.4) | 2 (14) | 0 | 4 (29) | 3 (21) | 5 (36) | 0 |
| 20-24 | 55 (2.4) | 5 (9) | 4 (7) | 13 (24) | 7 (13) | 21 (38) | 5 (9) |
| 25-29 | 81 (3.0) | 6 (7) | 5 (6) | 23 (28) | 14 (17) | 31 (38) | 2 (2) |
| 30-34 | 100 (4.0) | 12 (12) | 12 (12) | 23 (23) | 11 (11) | 38 (38) | 4 (4) |
| 35-39 | 41 (3.6) | 3 (7) | 5 (12) | 9 (22) | 5 (12) | 18 (44) | 1 (2) |
| ≥40 | 5 (3.0) | 1 (20) | 0 | 2 (40) | 2 (40) | 0 | 0 |
| Maternal race/ethnicity†: | |||||||
| Hispanic | 21 (0.9) | 0 | 1 (5) | 8 (38) | 3 (14) | 8 (38) | 1 (5) |
| Non-Hispanic black | 13 (1.2) | 1 (8) | 0 | 3 (23) | 1 (8) | 6 (46) | 2 (15) |
| Other | 15 (2.1) | 3 (20) | 1 (7) | 3 (20) | 2 (13) | 6 (40) | 0 |
| Non-Hispanic white | 249 (4.4) | 25 (10) | 24 (10) | 60 (24) | 36 (14) | 95 (38) | 9 (4) |
| Maternal education (years)†: | |||||||
| 0-8 | 4 (0.8) | 0 | 0 | 1 (25) | 1 (25) | 2 (50) | 0 |
| 9-11 | 27 (2.4) | 2 (7) | 0 | 7 (26) | 7 (26) | 9 (33) | 2 (7) |
| 12 | 66 (2.8) | 3 (5) | 6 (9) | 21 (32) | 9 (14) | 24 (36) | 3 (5) |
| 13-15 | 98 (3.8) | 13 (13) | 9 (9) | 19 (19) | 16 (16) | 37 (38) | 4 (4) |
| ≥16 | 102 (3.3) | 11 (11) | 11 (11) | 25 (25) | 9 (9) | 43 (42) | 3 (3) |
| Prepregnancy maternal body mass index (kg/m2)†: | |||||||
| <18.5 | 8 (1.6) | 1 (13) | 0 | 2 (25) | 3 (38) | 2 (25) | 0 |
| 18.5-24.9 | 162 (3.2) | 13 (8) | 14 (9) | 41 (25) | 24 (15) | 65 (40) | 5 (3) |
| 25-29.9 | 67 (3.1) | 11 (16) | 5 (7) | 18 (27) | 5 (7) | 23 (34) | 5 (7) |
| ≥30 | 60 (3.7) | 4 (7) | 7 (12) | 12 (20) | 10 (17) | 25 (42) | 2 (3) |
| Periconceptional maternal smoking: | |||||||
| No | 215 (2.7) | 21 (10) | 19 (9) | 59 (27) | 25 (12) | 83 (39) | 8 (4) |
| Yes | 82 (4.7) | 8 (10) | 7 (9) | 14 (17) | 17 (21) | 32 (39) | 4 (5) |
| Periconceptional maternal alcohol use: | |||||||
| No | 147 (2.4) | 16 (11) | 11 (7) | 31 (21) | 20 (14) | 64 (44) | 5 (3) |
| Yes | 149 (4.2) | 13 (9) | 14 (9) | 42 (28) | 22 (15) | 51 (34) | 7 (5) |
| Year of due date: | |||||||
| 1997-99 | 34 (2.0) | 0 | 0 | 11 (32) | 9 (26) | 14 (41) | 0 |
| 2000-01 | 36 (2.2) | 4 (11) | 0 | 14 (39) | 5 (14) | 11 (31) | 2 (6) |
| 2002-03 | 43 (2.8) | 7 (16) | 1 (2) | 9 (21) | 6 (14) | 17 (40) | 3 (7) |
| 2004-05 | 76 (4.5) | 2 (3) | 8 (11) | 18 (24) | 14 (18) | 31 (41) | 3 (4) |
| 2006-07 | 52 (3.1) | 5 (10) | 8 (15) | 13 (25) | 6 (12) | 19 (37) | 1 (2) |
| 2008-09 | 57 (3.6) | 11 (19) | 9 (16) | 9 (16) | 2 (4) | 23 (40) | 3 (5) |
*Sites are statewide for Arkansas, Iowa, and Utah. All other sites are selected regions. Years included: Arkansas 1998-2009, California 1997-2009, Georgia 1997-2009, Iowa 1997-2009, Massachusetts 1997-2009, New Jersey 1998-2003, New York 1997-2002 and 2004-09, North Carolina 2003-09, Texas 1997-2009, and Utah 2003-09.
†Variables were recoded to dichotomous categories for adjusted analyses; non-Hispanic white versus other, 0-12 years education versus >12 years, body mass index <30 versus ≥30 kg/m2.
In a bayesian analysis of the current NBDPS data that takes into account non-NBDPS individual drug-specific birth defect associations previously reported in the literature, no association with maternal use of citalopram or escitalopram monotherapy was found, except for a marginal association between citalopram and neural tube defects (table 3). For fluoxetine treatment, associations were seen for ventricular septal defects, right ventricular outflow tract obstruction cardiac defects, and craniosynostosis. Paroxetine had the most previously reported associations, and significant associations were observed for five of the seven defects assessed. Associations between paroxetine and anencephaly, atrial septal defects, and right ventricular outflow tract obstruction cardiac defects found in other studies were confirmed in this independent dataset, and two other associations seen in the previous NBDPS analysis8 (gastroschisis and omphalocele) were again seen in this analysis. For sertraline, the most commonly used SSRI in our study, the findings for all five defects assessed were not significant.
Table 3.
Odds ratios and 95% confidence interval, resulting prior odds ratio, and bayesian complete case analysis posterior odds ratio with 95% credible interval, National Birth Defects Prevention Study (NBDPS), 1997-2009
| Birth defect by SSRI | Odds ratio (95% CI) | Prior odds ratio (95% CI)* | Odds ratio (95%CI): Alwan et al8 | Posterior odds ratio (95% CrI): NBDPS 1997-2009 | ||
|---|---|---|---|---|---|---|
| Louik et al11 | Bakker et al9, Kornum et al10, Reis and Kallen13 | Malm et al12 | ||||
| Citalopram: | ||||||
| Neural tube defects | — | — | 2.5 (1.2 to 5.1) (n=8) | 2.5 (1.2 to 5.1) | — | 1.8 (1.0 to 3.0) (n=5) |
| Ventricular septal defects | — | — | 1.3 (0.9 to 1.8) (n=36) | 1.3 (0.9 to 1.8) | — | 1.3 (0.9 to 1.8) (n=6) |
| Cleft lip with or without cleft palate | 3.2 (0.9 to 11.9) (n=4) | — | — | 3.2 (0.9 to 11.9) | — | 1.4 (0.7 to 2.7) (n=8) |
| Hypospadias | 1.9 (0.4 to 8.8) (n=4) | 1.3 (0.9 to 1.8)13 (n=38) | — | 1.3 (0.6 to 2.5 | — | 1.2 (0.7 to 2.0) (n=8) |
| Escitalopram: | ||||||
| Septal defects | 4.2 (1.0 to 17.1)10 (n=3) | 1.5 (0.7 to 3.0) (n=8)† | 1.7 (0.7 to 3.7) | 1.3 (0.7 to 2.1) (n=11) | ||
| Fluoxetine: | ||||||
| Ventricular septal defects | — | — | 1.5 (1.0 to 2.2) (n=26) | 1.5 (1.0 to 2.2) | — | 1.4 (1.0 to 1.9) (n=16) |
| RVOTO | 1.0 (0.2 to 3.4) (n=4) | — | 2.7 (0.9 to 8.5) (n=3) | 1.5 (0.6 to 3.6) | 0.9 (0.3 to 2.7) (n=4) | 2.0 (1.4 to 3.1) (n=27) |
| Esophageal atresia | — | — | — | Non-informative prior | 2.4 (0.9 to 6.4) (n=5) | 1.8 (0.8 to 3.4) (n=10) |
| Craniosynostosis | — | — | — | Non-informative prior | 2.8 (1.3 to 6.1) (n=10) | 1.9 (1.1 to 3.0) (n=21) |
| Paroxetine: | ||||||
| Anencephaly | Neural tube defect: 3.3 (1.1 to 10.4) (n=4) | — | — | 3.3 (1.1 to 10.4) | 5.1 (1.7 to 15.3) (n=5) | 3.2 (1.6 to 6.2) (n=6) |
| Atrial septal defects | 5.7 (1.4 to 23.7)9 (n=3) | 1.3 (0.4 to 4.0) (n=3) | 1.8 (0.7 to 4.5) | — | 1.8 (1.1 to 3.0) (n=15) | |
| RVOTO | 3.3 (1.3 to 8.8) (n=6) | — | 5.2 (1.6 to 16.3) (n=3) | 3.9 (1.4 to 10.4) | 2.5 (1.0 to 6.0) (n=7) | 2.4 (1.4 to 3.9) (n=16) |
| Cleft palate | 1.5 (0.4 to 5.3) (n=3) | — | 2.7 (1.0 to 7.1) (n=4) | 1.7 (0.7 to 4.0) | 1.7 (0.6 to 4.8) (n=5) | 1.3 (0.7 to 2.3) (n=10) |
| Hypospadias | 1.0 (0.3 to 3.3) (n=3) | 2.5 (1.1 to 4.6)13 (n=9) | — | 1.6 (0.6 to 3.4) | 0.6 (0.2 to 2.4) (n=3) | 1.1 (0.6 to 1.9) (n=9) |
| Gastroschisis | — | — | — | Non-informative prior | 2.9 (1.0 to 8.4) (n=5) | 2.5 (1.2 to 4.8) (n=13) |
| Omphalocele | — | — | — | Non-informative prior | 8.1 (3.1 to 20.8) (n=6) | 3.5 (1.3 to 8.0) (n=6) |
| Sertraline: | ||||||
| Anencephaly | 0.8 (0.1 to 6.3) (n=1) | — | — | 0.8 (0.1 to 6.3) | 3.2 (1.1 to 9.3) (n=4) | 1.2 (0.5 to 2.5) (n=7) |
| Septal defects | 2.0 (1.2 to 4.0) (n=13) | 3.3 (1.5 to 7.5)10 (n=6) | 0.5 (0.2 to 1.3) (n=5)† | 1.5 (0.6 to 2.9) | 0.7 (0.3 to 1.5) (n=10) | 1.0 (0.8 to 1.4) (n=47) |
| Anal atresia | 4.4 (1.2 to 16.4) (n=3) | — | — | 4.4 (1.2 to 16.4) | 0.7 (0.2 to 2.8) (n=4) | 1.4 (0.8 to 2.3) (n=11) |
| Any limb reduction | 3.9 (1.1 to 13.5) (n=3) | — | — | 3.9 (1.1 to 13.5) | Transverse: 1.2 (0.4 to 4.0) (n=3) | 1.2 (0.7 to 2.0) (n=13) |
| Omphalocele | 5.7 (1.6 to 20.7) (n=3) | — | — | 5.7 (1.6 to 20.7) | 1.5 (0.4 to 6.6) (n=3) | 1.4 (0.7 to 2.8) (n=4) |
SSRI=selective serotonin reuptake inhibitor; RVOTO=right ventricular outflow tract obstruction cardiac defects.
Adjusted for maternal race/ethnicity, maternal education, obesity, and smoking.
*Priors are only based on studies described in first three column and exclude Alwan et al.8 No informative prior was calculated if the only available data were from Alwan et al, but this association was reported in that earlier analysis using a subset of NBDPS data.
†Ventricular septal defect.
A bayesian analysis using a non-informative prior (that is, assuming there were no previously published studies to help develop an informative prior), and sensitivity analyses using bayesian methods in which missing data for confounders were replaced with imputed values showed similar results for most associations except for the association between citalopram and neural tube defects. The prior for that association was based on one cohort study, which found a higher odds ratio, and our logistic regression analysis without considering this prior study showed no association (see supplementary table).
Discussion
Using data from the US National Birth Defects Prevention Study (NBDPS), we confirmed previously reported associations between right ventricular outflow tract obstruction cardiac defects in infants and maternal use of fluoxetine12 or paroxetine8 11 12 early in pregnancy, and between anencephaly8 11 or atrial septal defects9 in infants and maternal use of paroxetine. This analysis also confirmed associations between gastroschisis or omphalocele and paroxetine and between craniosynostosis and fluoxetine that were reported in the analysis of an earlier subset of NBDPS data8; however, these still require corroboration in an independent data source. It is reassuring that none of the five previously reported associations between sertraline and birth defects8 10 11 12 were confirmed in this analysis, particularly since about 40% of women reporting use of an SSRI in early pregnancy used sertraline. In addition, we did not find support for nine other previously reported associations between maternal SSRI treatment and selected birth defects in the child.
Although our analysis strongly supports the validity of the associations that were observed, the increase in the absolute risks, if the associations are causal, is small. The two strongest posterior odds ratios were seen for maternal paroxetine treatment and anencephaly (3.2) or right ventricular outflow tract obstruction cardiac defects (2.4) in the infant. If these associations are causal, the absolute risks in the children of women who are treated with paroxetine early in pregnancy would increase for anencephaly from 2 per 10 00024 to 7 per 10 000, and for right ventricular outflow tract obstruction cardiac defects from 10 per 10 00025 to 24 per 10 000. The absolute risks for these birth defects are still low.
This analysis confirms the need to assess the association between specific SSRIs and specific birth defects rather than combining an entire drug class or heterogeneous group of birth defects. Although SSRIs are similar pharmacologically, there are chemical differences, and if any of them do have teratogenic activity, it may be completely unrelated to the inhibition of serotonin receptors. SSRIs also differ pharmacokinetically,26 and this could account for differences in teratogenic activity, whether or not the mechanism involved inhibition of serotonin receptors.27
Limitations of this study
This analysis does not address whether the birth defect associations we observed were caused by maternal SSRI treatment, underlying maternal disease, or some other factor. Since there was no specific question on depression and we cannot identify all participants with untreated depression, there is the possibility of confounding by indication.
A recent publication by Furu and colleagues combined data from five Nordic countries.28 Some overlap occurred between the data included in this recent study and the three Nordic studies we included in our meta-analysis, but this study also included data from Norway and Iceland that is not included in our analyses.10 12 13 Many of the associations we assessed for septal heart defects and right ventricular outflow tract obstruction cardiac defects showed similar risk estimates in our analysis and the recent study. One clear different finding is the association reported by Furu and colleagues for anal atresia and sertraline that was also reported by Louik and colleagues but is not evident in the NBDPS data.11 28
Additional limitations of this analysis need to be acknowledged. Periconceptional exposure was based on maternal self report, with interviews conducted six weeks to 24 months after the expected date of delivery. However, Kwon and colleagues reported good concordance between self report of antidepressants and claims data.29
We made 21 comparisons between exposure and outcome using five different models, and it is possible that some statistically significant findings occurred owing to the occurrence of false positive associations expected with multiple comparisons.30 Another limitation is that the small numbers for some of the individual birth defects and some of the specific exposures resulted in unstable estimates. Finally, exposure ascertainment is known to be more complete when women are specifically asked about their use of drugs by name31; the interview did not include a specific question about use of escitalopram.
Strengths of this study
This analysis also has some important strengths. The bayesian analysis enabled consideration of evidence both for and against an association between use of SSRIs and risk of birth defects from previous epidemiological studies in the analyses and used relatively homogenous and discrete classes of birth defects and SSRI monotherapy. As a result, our study provides strong evidence for the reproducibility and validity of the associations that were observed. The sensitivity assessment showed that missing data were unlikely to have affected the results. The association between SSRIs and heart defects is biologically plausible; Sadler27 has suggested that the association may be a result of the key role that the neurotransmitter serotonin (5-hydroxytryptamine) plays in embryonic development of the heart.
A major advantage of this analysis over some previous reports is the ability to assess individual SSRIs and individual birth defects, while accounting for earlier reported associations. Although the data are self reported, they do represent reported use of the medications and not just filling of the prescription. Approximately 30% of mothers stop taking SSRIs during pregnancy,32 and this might not be captured if we relied on prescription information or medical records, since prescriptions might have been filled but not taken after the pregnancy was recognized. We have previously used NBDPS data to show that antidepressant prescription patterns have changed over time,5 making it more challenging to study these associations.
Our analyses of a large population based case-control dataset combined with prior odds ratios based on the literature allowed us to show and refine associations between maternal fluoxetine or paroxetine treatment during pregnancy and right ventricular outflow tract obstruction cardiac defects and between maternal use of paroxetine and anencephaly or atrial septal defects. In contrast, we found no evidence to support 14 other previously reported associations between maternal SSRI use and birth defects. Continued scrutiny of the association between SSRIs and birth defects is warranted, and additional studies of specific SSRI treatments during pregnancy and birth defects are needed to enable women and their healthcare providers to make more informed decisions about treatment. Meanwhile, the current analysis can help guide healthcare providers and women to the safest options for treatment during early pregnancy to minimize the risk of major birth defects, while providing adequate treatment of maternal depression.
What is already known on this topic
Selective serotonin reuptake inhibitors (SSRIs) are increasingly used by women of reproductive age and during pregnancy
However, inconsistent reports on the association with birth defects have limited opportunities for clinicians to carefully evaluate the risk compared with benefit of specific SSRIs during pregnancy
What this study adds
This study combined summarized results from published literature with data from the National Birth Defects Prevention Study using bayesian analysis
It showed consistent results for 7 of 21 evaluated associations between specific SSRIs and birth defects
We thank Tiffany Colarusso and Jennifer Lind for their valuable contributions to this paper. Coding of drug information in the National Birth Defects Prevention Study used the Slone Drug Dictionary under license from the Slone Epidemiology Center of Boston University.
Contributors: JR designed the study, did the frequentist data analyses, and wrote the first draft of the manuscript. She is the guarantor. OD performed the bayesian statistical analyses and revised the drafted manuscript. JMF, CL, and MAH provided epidemiological feedback on initial data tables and revised the drafts. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Funding: Data collection was funded by the US Centers for Disease Control and Prevention.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: no support from any organisation for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Ethical approval: This study was approved by the institutional review board of the Centers for Disease Control and Prevention and all participating sites.
Data sharing: Previous publications with a subset of this data: Alwan S, Reefhuis J, Rasmussen SA, et al. Use of selective serotonin-reuptake inhibitors in pregnancy and the risk of birth defects. N Engl J Med 2007;356:2684-92 and Alwan S, Reefhuis J, Rasmussen SA, et al. Patterns of antidepressant medication use among pregnant women in a United States population. J Clin Pharmacol 2011;51:264-70.
Transparency: The leader author (JR) hereby affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Cite this as: BMJ 2015;350:h3190
Web Extra. Extra material supplied by the author
Appendix table: Odds ratios for associations between selected SSRIs and birth defects using different statistical methods
Appendix: statistical methods
References
- 1.FDA. FDA advising of risk of birth defects with paxil. 2005. www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2005/ucm108527.htm.
- 2.Gentile S. Selective serotonin reuptake inhibitor exposure during early pregnancy and the risk of birth defects. Acta Psychiatr Scand 2011;123:266-75. [DOI] [PubMed] [Google Scholar]
- 3.Wurst KE, Poole C, Ephross SA, et al. First trimester paroxetine use and the prevalence of congenital, specifically cardiac, defects: a meta-analysis of epidemiological studies. Birth Defects Res A Clin Mol Teratol 2010;88:159-70. [DOI] [PubMed] [Google Scholar]
- 4.Goodman JH. Women’s attitudes, preferences, and perceived barriers to treatment for perinatal depression. Birth 2009;36:60-9. [DOI] [PubMed] [Google Scholar]
- 5.Alwan S, Reefhuis J, Rasmussen SA, et al. Patterns of antidepressant medication use among pregnant women in a United States population. J Clin Pharmacol 2011;51:264-70. [DOI] [PubMed] [Google Scholar]
- 6.Huybrechts KF, Palmsten K, Mogun H, et al. National trends in antidepressant medication treatment among publicly insured pregnant women. Gen Hosp Psychiatry 2013;35:265-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tinker SC, Broussard CS, Frey MT, et al. Prevalence of prescription medication use among non-pregnant women of childbearing age and pregnant women in the United States: NHANES, 1999-2006. Matern Child Health J 2015;19:1097-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alwan S, Reefhuis J, Rasmussen SA, et al. Use of selective serotonin-reuptake inhibitors in pregnancy and the risk of birth defects. N Engl J Med 2007;356:2684-92. [DOI] [PubMed] [Google Scholar]
- 9.Bakker MK, Kerstjens-Frederikse WS, Buys CH, et al. First-trimester use of paroxetine and congenital heart defects: a population-based case-control study. Birth Defects Res A Clin Mol Teratol 2010;88:94-100. [DOI] [PubMed] [Google Scholar]
- 10.Kornum JB, Nielsen RB, Pedersen L, et al. Use of selective serotonin-reuptake inhibitors during early pregnancy and risk of congenital malformations: updated analysis. Clin Epidemiol 2010;2:29-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Louik C, Lin AE, Werler MM, et al. First-trimester use of selective serotonin-reuptake inhibitors and the risk of birth defects. N Engl J Med 2007;356:2675-83. [DOI] [PubMed] [Google Scholar]
- 12.Malm H, Artama M, Gissler M, et al. Selective serotonin reuptake inhibitors and risk for major congenital anomalies. Obstet Gynecol 2011;118:111-20. [DOI] [PubMed] [Google Scholar]
- 13.Reis M, Kallen B. Delivery outcome after maternal use of antidepressant drugs in pregnancy: an update using Swedish data. Psychol Med 2010;40:1723-33. [DOI] [PubMed] [Google Scholar]
- 14.Gelman A, Shalizi CR. Philosophy and the practice of Bayesian statistics. Br J Math Stat Psychol 2013;66:8-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoon PW, Rasmussen SA, Lynberg MC, et al. The National Birth Defects Prevention Study. Public Health Rep 2001;116(Suppl 1):32-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rasmussen SA, Olney RS, Holmes LB, et al. Guidelines for case classification for the National Birth Defects Prevention Study. Birth Defect Res A Clin Mol Teratol 2003;67:193-201. [DOI] [PubMed] [Google Scholar]
- 17.Reefhuis J, Gilboa SM, Anderka M, et al. The national birth defects prevention study: a review of the methods. Birth Defect Res A Clin Mol Teratol 2015; published online 2 Jun. [DOI] [PMC free article] [PubMed]
- 18.Correa A, Gilboa SM, Besser LM, et al. Diabetes mellitus and birth defects. Am J Obstet Gynecol 2008;199:237 e1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Obican S, Scialli AR. Teratogenic exposures. Am J Med Genet Part C 2011;157C:150-69. [DOI] [PubMed]
- 20.Sutton AJ, Abrams KR. Bayesian methods in meta-analysis and evidence synthesis. Stat Methods Med Res 2001;10:277-303. [DOI] [PubMed] [Google Scholar]
- 21.Lunn D, Jackson C, Best N, et al. The BUGS book: a practical introduction to Bayesian analysis, CRC Press, 2012.
- 22.Abuhamad AZ. ACOG Practice Bulletin, clinical management guidelines for obstetrician-gynecologists number 98, October 2008 (replaces Practice Bulletin No 58, Dec 2004). Ultrasonography in pregnancy. Obstet Gynecol 2008;112:951-61. [DOI] [PubMed] [Google Scholar]
- 23.Practice ACoO. ACOG committee opinion No 354: treatment with selective serotonin reuptake inhibitors during pregnancy. Obstet Gynecol 2006;108:1601-3. [DOI] [PubMed] [Google Scholar]
- 24.Parker SE, Mai CT, Canfield MA, et al. Updated national birth prevalence estimates for selected birth defects in the United States, 2004-2006. Birth Defects Res A Clin Mol Teratol 2010;88:1008-16. [DOI] [PubMed] [Google Scholar]
- 25.Bedard T, Lowry RB, Sibbald B, et al. Folic acid fortification and the birth prevalence of congenital heart defect cases in Alberta, Canada. Birth Defects Res A Clin Mol Teratol 2013;97:564-70. [DOI] [PubMed] [Google Scholar]
- 26.Edwards JG, Anderson I. Systematic review and guide to selection of selective serotonin reuptake inhibitors. Drugs 1999;57:507-33. [DOI] [PubMed] [Google Scholar]
- 27.Sadler TW. Selective serotonin reuptake inhibitors (SSRIs) and heart defects: potential mechanisms for the observed associations. Reprod Toxicol 2011;32:484-9. [DOI] [PubMed] [Google Scholar]
- 28.Furu K, Kieler H, Haglund B, et al. Selective serotonin reuptake inhibitors and venlafaxine in early pregnancy and risk of birth defects: population based cohort study and sibling design. BMJ 2015;350:h1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kwon A, Bungay KM, Pei Y, et al. Antidepressant use: concordance between self-report and claims records. Med Care 2003;41:368-74. [DOI] [PubMed] [Google Scholar]
- 30.Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology 1990;1:43-6. [PubMed] [Google Scholar]
- 31.Mitchell AA, Cottler LB, Shapiro S. Effect of questionnaire design on recall of drug exposure in pregnancy. Am J Epidemiol 1986;123:670-6. [DOI] [PubMed] [Google Scholar]
- 32.Reefhuis J, Rasmussen SA, Friedman JM. Selective serotonin-reuptake inhibitors and persistent pulmonary hypertension of the newborn. N Engl J Med 2006;354:2188-90; author reply 88-90. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix table: Odds ratios for associations between selected SSRIs and birth defects using different statistical methods
Appendix: statistical methods