Table 3.
Steric and electronic influence on the relative rates for the reduction of esters.
| Entry | 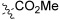 |
RV[a] |
|---|---|---|
| 1 | 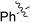 |
>100 |
| 2 | 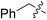 |
9.14 |
| 3 | 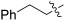 |
4.29 |
| 4 | 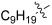 |
1.00 |
| 5 | 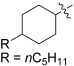 |
0.41 |
| 6 | 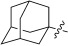 |
0.26 |
| 7 | 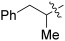 |
0.91 |
| 8 | 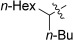 |
0.05 |
| Entry | 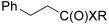 |
RV[a] |
|---|---|---|
| 9 | R=OMe | 1.00 |
| 10 | R=OPh | 6.88 |
| 11 | R=Opfp | 9.15 |
| 12 | R=SEt | 5.78 |
Relative reactivity values (RV) determined from product distribution by 1H NMR and/or GC analyses of crude reaction mixtures. All data represent the average of at least two experiments. pfp=pentafluorophenyl.
